
A WORD FROM OUR CHIEF SCIENTIFIC OFFICER
Dear FAPI community,
As many of you know, in October, SOFIE Biosciences and GE Healthcare signed a global licensing agreement for the development and commercialization of SOFIE’s two investigational Gallium-68 and Fluorine-18 Fibroblast Activation Protein Inhibitors (FAPI) radiopharmaceutical tracers – [68Ga]FAPI-46 and outside-US rights for [18F]FAPI-74. Based on this agreement, GE HealthCare will take on global rights for [68Ga]FAPI-46 and outside-US rights for [18F]FAPI-74, originally developed at Heidelberg University in Germany, and both currently in Phase II clinical trials in the U.S. ([68Ga]FAPI-46 (NCT05262855) and [18F]FAPI-74 (NCT05641896)). SOFIE will continue its clinical development and commercialization program with [18F]FAPI-74 in the U.S.
This partnership comes at an ideal time, building on the momentum created by the SOFIE-sponsored clinical trials well underway and the vast international reach of the FAPI Global Outreach Program. Our shared goal with GEHC is to develop FAP imaging products through continued clinical trials and towards regulatory submission, and potential commercialization.
We continue to make progress in the recruitment of patients as part of the Phase 2 prospective clinical trials with [68Ga]FAPI-46 and [18F]FAPI-74. Additional information is available in the “FAPI Clinical Development Updates” below.
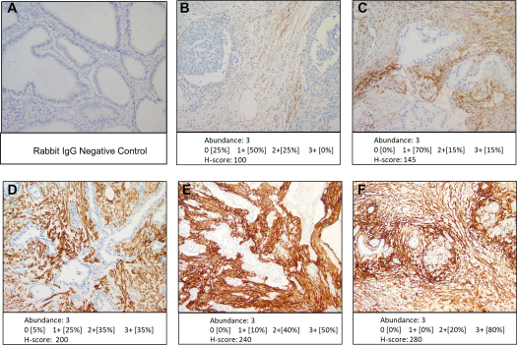
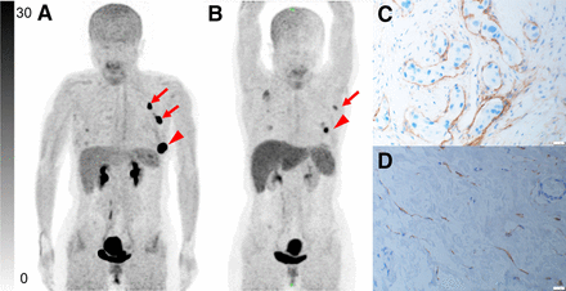
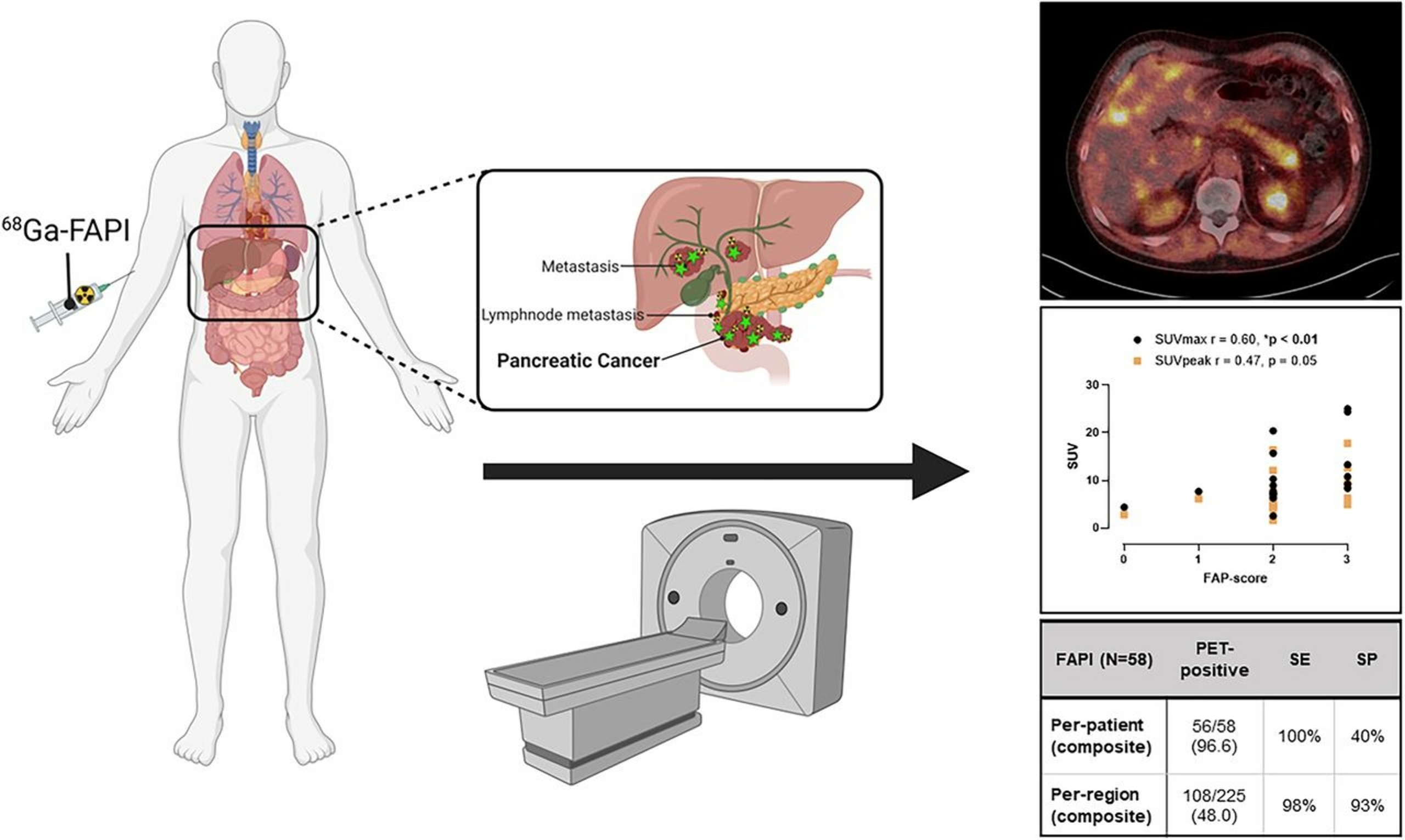
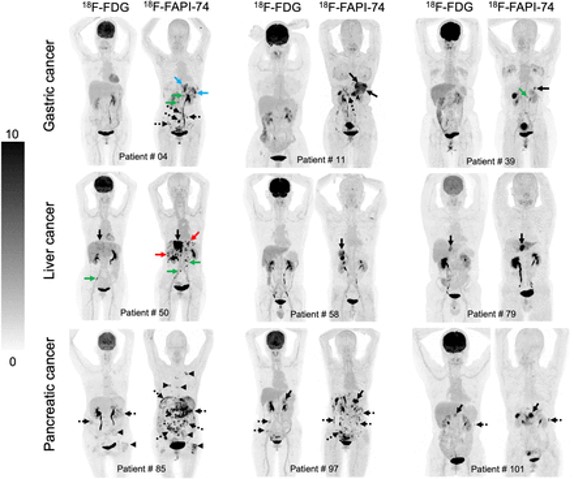
Also of note is our recent publication in PloS One, through our collaboration with our colleagues, specifically at Mayo Clinic. This paper, included in our publication highlights below, features the [68Ga]FAPI-46 Phase 2 clinical study.
Thank you to our FAPI Global Outreach Program members who submitted their annual progress reports. We’ve gleaned important information that will help shape the program moving forward.
As always, thank you for reading our newsletter. We sincerely appreciate your input on how this can be a better resource for the FAPI community.

FAPI CLINICAL DEVELOPMENT UPDATES

PUBLICATION HIGHLIGHTS
Each newsletter will feature recent FAPI publications.
FAPI GLOBAL OUTREACH PROGRAM ANNUAL PROGRESS REPORT
A special thanks to our FAPI Global Outreach Program members for submitting their annual reports! Based on the results, 1,700 patients have been scanned in our program, 14 grants have been received, almost 40 of you have published or will within the next six months and 18 have presented at scientific conferences. We appreciate this partnership and wish you all the best with your research progress in utilizing FAPI.
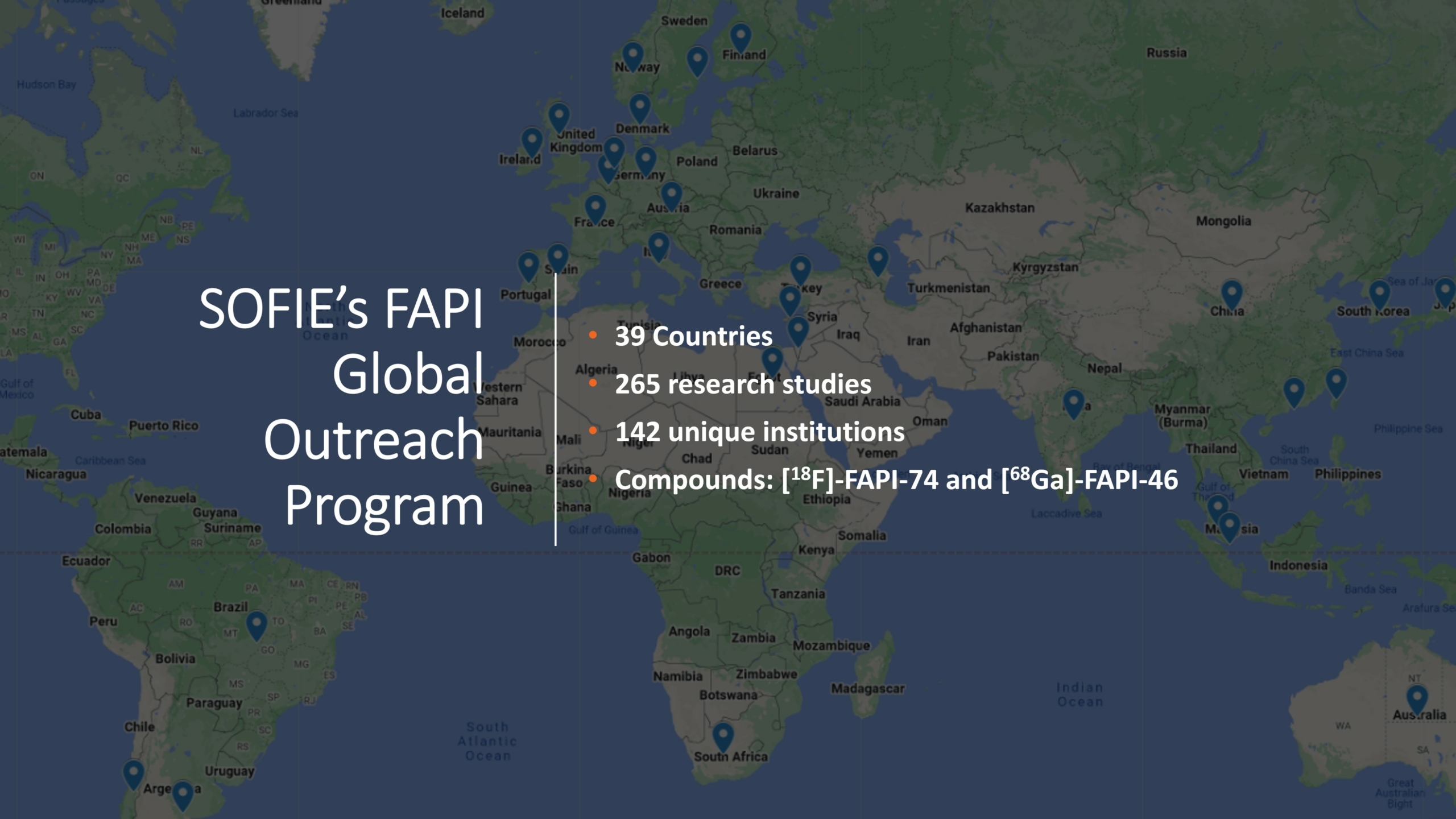
SOFIE has engaged with institutions globally to unlock FAPI’s clinical utility potential.
Learn more about how you can participate.




