
A WORD FROM OUR CHIEF SCIENTIFIC OFFICER
Dear FAPI Community,
As the year comes to a close, I’m proud to share the whirlwind of activity in the FAPI space over the last several months.
Last month, I participated in three forums that highlighted FAP advancements. On November 7, I was invited to a roundtable discussion sponsored by Perceptive and Lantheus on “FAPI-PET imaging & theranostics: A new era in targeting cancer and fibrotic diseases”. SOFIE was a silver sponsor of the next two events, the 2024 Associate Technical Affiliates of Western Michigan (ATAWM) and Central Chapter Society of Nuclear Medicine and Molecular Imaging (CCSNMMI) Michigan Road Show and the ICPO Theranostics Virtual Summit . It is a pleasure and privilege to be a part of these events so critical to broadening and educating our FAPI community and, importantly, challenge our perspectives.
Our 18F-FAPI-74 Phase 2 clinical study in gastrointestinal cancers in nearing completion and our team is working diligently on Phase 3 planning, which , pending final FDA review, is expected to be launched in late 2025.
We would like to thank our Global Outreach Program community for submitting your annual Progress Reports. It has been instructive to learn more about your research programs utilizing FAPI, and we wish you continued progress in your efforts.
As always, thank you for reading our newsletter. We value your suggestions on how this newsletter can become a better resource for you. Wishing everyone a wonderful holiday season and a happy new year!

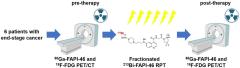
![Evaluation of Targeted Alpha Therapy Using [211At]FAPI1 in Triple-Negative Breast Cancer Xenograft Models Evaluation of Targeted Alpha Therapy Using [211At]FAPI1 in Triple-Negative Breast Cancer Xenograft Models](https://sofie.com/wp-content/uploads/2024/12/thumbnail-small-1.jpg)
Int J Mol Sci. 2024 Oct 28
![Impact of fat intake on [18F]AIF-NOTA-FAPI-04 uptake in normal abdominal organs Impact of fat intake on [18F]AIF-NOTA-FAPI-04 uptake in normal abdominal organs](https://sofie.com/wp-content/uploads/2024/12/thumbnail-small.jpg)
Impact of fat intake on [18F]AIF-NOTA-FAPI-04 uptake in normal abdominal organs
Front Med (Lausanne). 2024 Nov 7
FAPI OUTREACH PROGRAM
Join us in advancing the frontier of molecular imaging through the FAPI Outreach Program
SOFIE is continuing to accept applications for our FAPI Outreach Program. This program aims to raise awareness and foster collaboration in the field of molecular imaging, specifically focusing on Fibroblast Activation Protein Inhibitor (FAPI) studies. This program seeks to engage with healthcare professionals, researchers, and the broader community to share knowledge, advancements, and the potential impact of FAPI in diagnostic and therapeutic applications.
*GE Healthcare licensed products and regions will be ready for new application reviews in April.






