OVERVIEW
PIPELINE
In Nov 2019, SOFIE Biosciences (SOFIE) licensed quinoline-based PET tracers, developed by the team at Heidelberg University, that act as FAP inhibitors (FAPI) and showed promising clinical data in a variety of oncologic and non oncologic diseases. SOFIE subsequently led the development of the lead gallium 68 product ([68Ga]FAPI-46) along with the lead fluorine 18 product ([18F]FAPI-74), in Phase 2 clinical studies in the US, and launched the FAPI Outreach Program.
In October 2023, SOFIE and GE Healthcare (GEHC) entered a licensing agreement to develop, manufacture and commercialize [68Ga]FAPI-46 and [18F]FAPI-74 for diagnostic and companion diagnostic use. In this agreement, GE HealthCare will take on global rights for [68Ga]FAPI-46 and outside-US rights for [18F]FAPI-74. SOFIE will maintain clinical development and commercialization rights for [18F]FAPI-74 in the US.
Building on the momentum created by SOFIE’s sponsored trials in the US and the FAPI Global Outreach Program which engages 150 academic institutions in 39 countries to unlock FAPI’s clinical utility, GEHC aims to develop FAP imaging products through clinical trials and towards regulatory submission, and potential commercialization in various regions. SOFIE and GEHC will collaborate on both the development and commercialization processes through a joint steering committee.
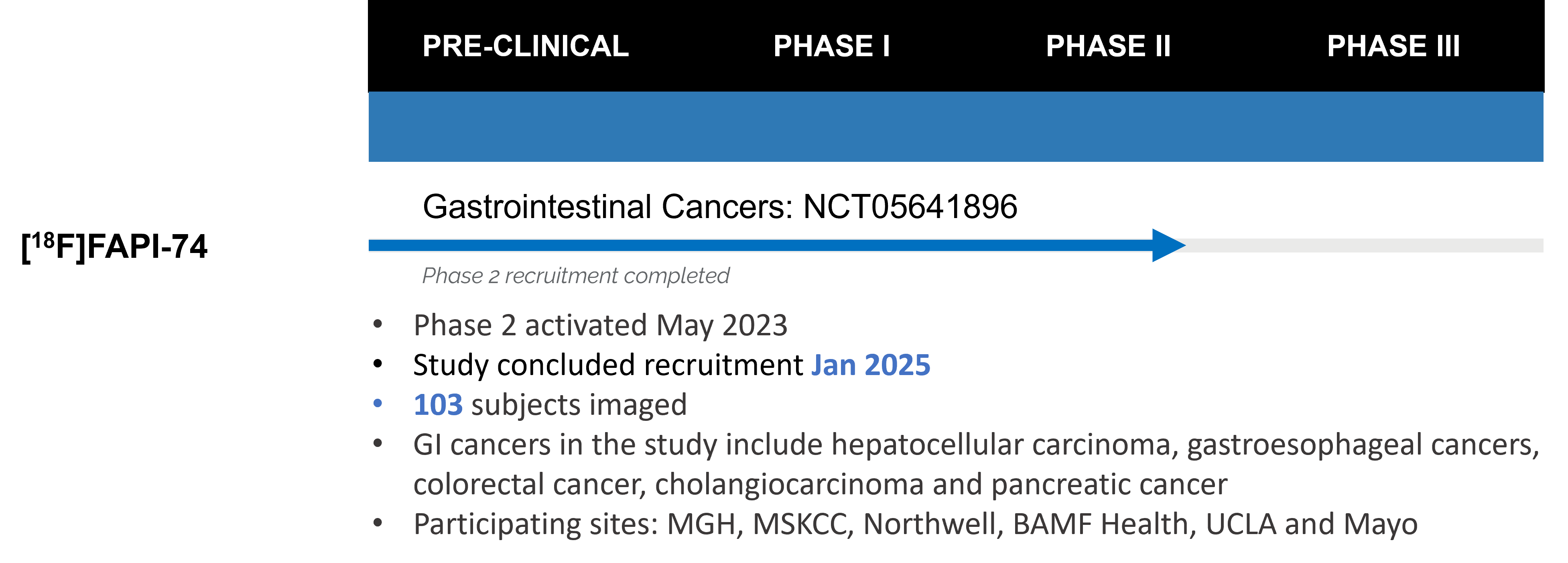
In the US, [68Ga]FAPI-46 is in a Phase 2 clinical trial in patients with Pancreatic Ductal Adenocarcinoma. In addition, [18F]FAPI-74 has entered a Phase 2 clinical trial in patients with cancers of the GI tracts. Both Phase 2 studies are anticipated to conclude in 2024.
DEVELOP AND DELIVER
Staying true to our vision to improve patient outcomes by developing and delivering molecular diagnostics and therapeutics, SOFIE has acquired global rights for the diagnostic and companion diagnostic use of the FAPI family of compounds from University of Heidelberg, the team that invented PSMA-617 (Novartis). The FAPI family of compounds have demonstrated promising theranostic potential.
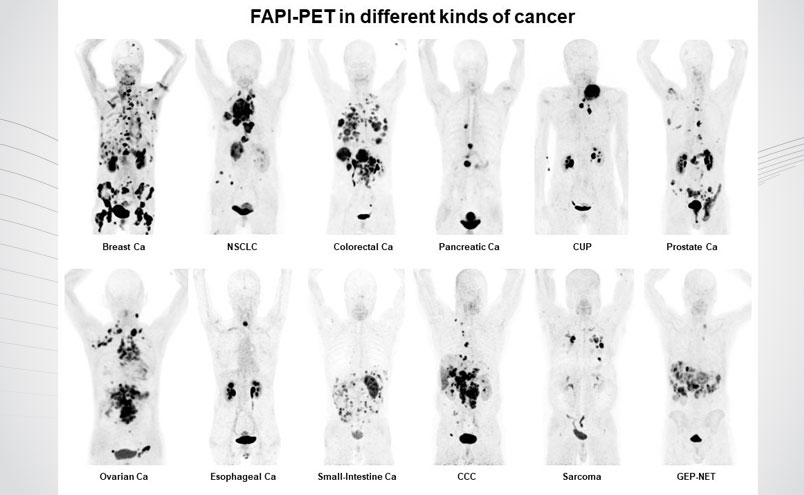
Fibroblast activation protein (FAP) is a well-known target shown to be overexpressed on cancer associated fibroblasts (CAFs) present in the tumor microenvironment across a variety of cancer types. In addition to oncology, FAP overexpression has been linked to non-malignant processes and disease, including wound healing, chronic inflammation, myocardial infarction, benign tumors and fibrosis. The FAP inhibitor family of compounds (FAPI) allows non-invasive visualization of this important target for oncology and non-oncology indications through binding to FAP with low nanomolar affinity. These compounds can be labeled with various isotopes, including Gallium-68 and Florine-18, for use in PET imaging.
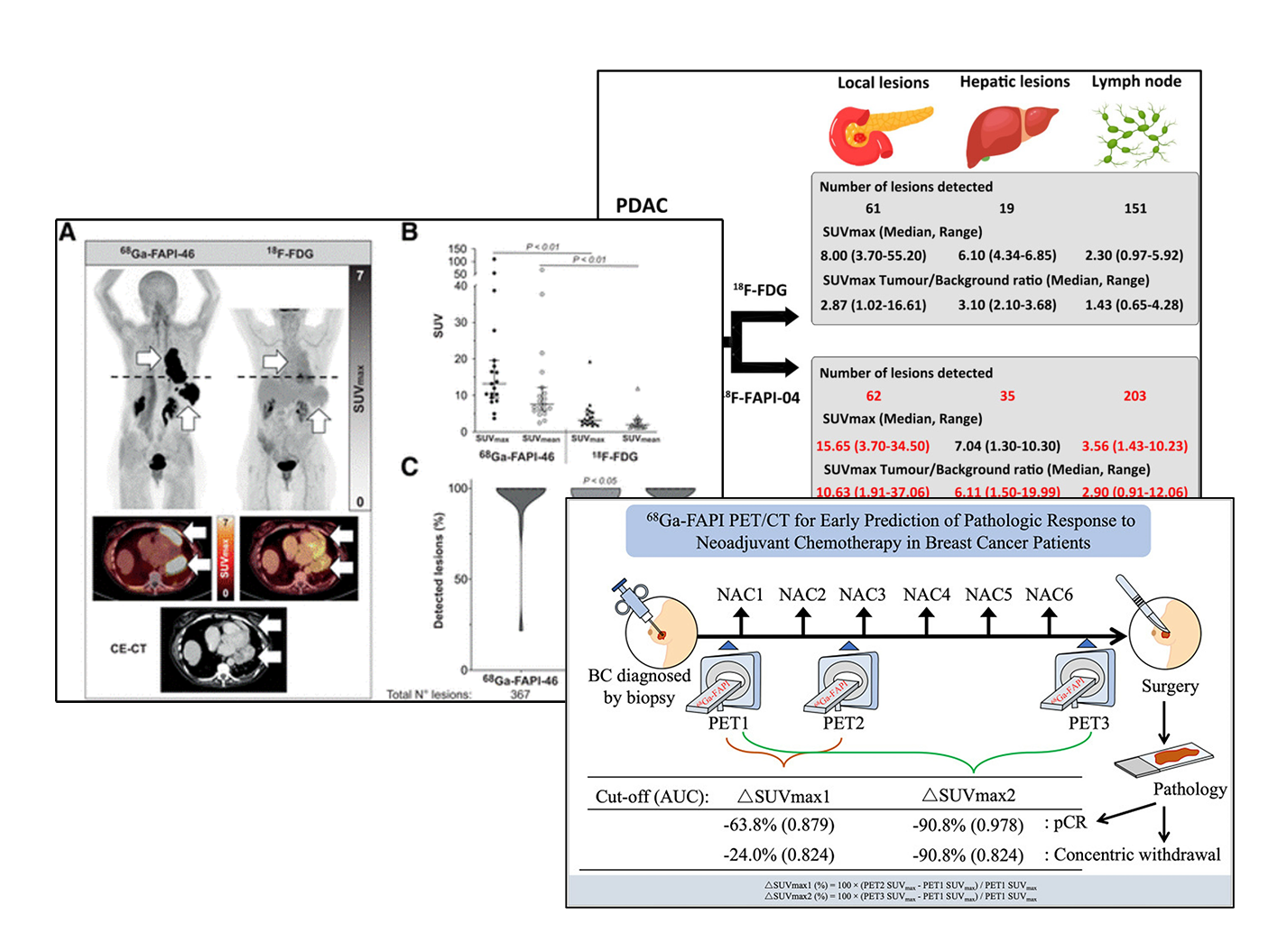
SOFIE is actively pursuing the clinical development of two lead compounds: FAPI-46 as its lead Ga labelled and FAPI-74 as its 18F labelled compound. Both have demonstrated favorable biodistribution and dosimetry, target-specific uptake, excellent tumor to background ratio, and no toxicity observed in animal models and human subjects. The FAPI compounds have been published in over 2,000 patients showing equal or superior performance over FDG or standard of care in various cancer indications.