A WORD FROM OUR CHIEF SCIENTIFIC OFFICER
Dear FAPI Community,
For our first newsletter of 2025, I am happy to share that things are off to a roaring start on the FAPI front.
But first – a final highlight of 2024 was the activation of [18F]FAPI-74 production at now FIVE sites in SOFIE’s network – Totowa, NJ; Gilroy, CA; Romeoville, IL; Sanford, FL, and Cleveland, OH. This growth in FAPI-74 manufacturing capability uniquely positions us to support our upcoming [18F]FAPI-74 clinical studies and academic investigator-initiated studies, in addition to companion use of [18F]FAPI-74 in Pharma collaborations. Additional sites are planned for FAPI-74 manufacturing in 2025 so be sure to follow SOFIE’s LinkedIn account for the latest.
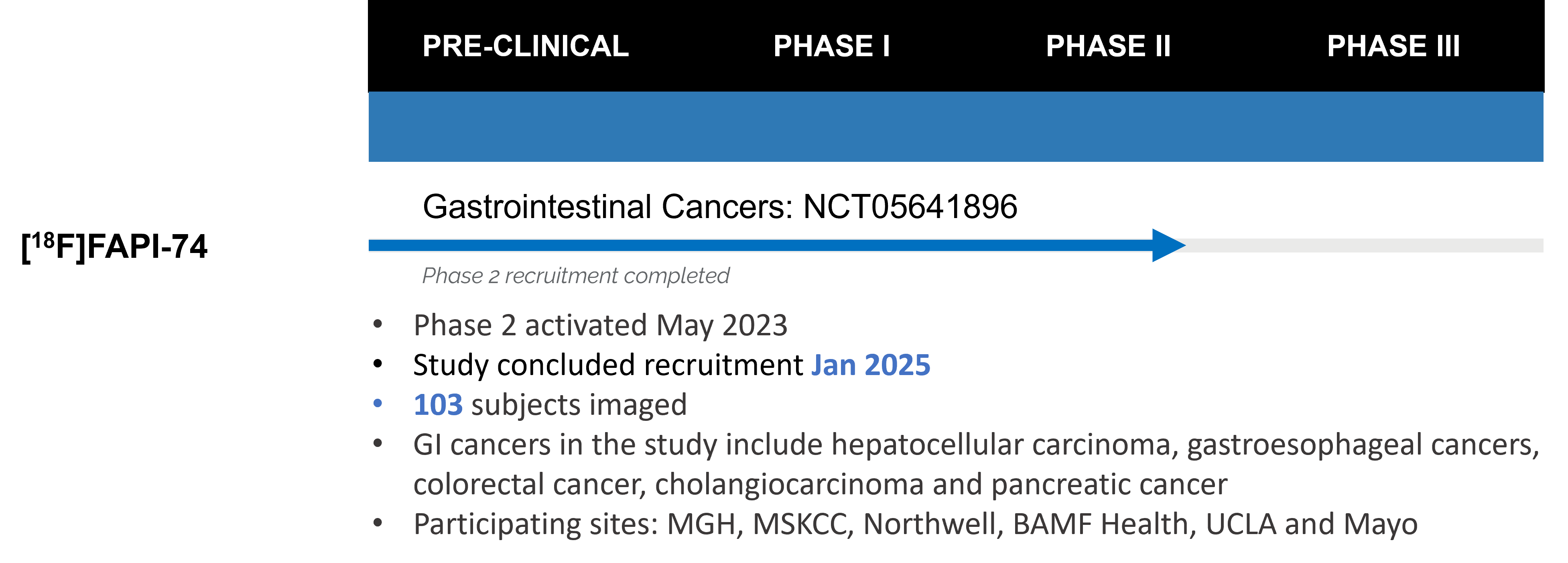
We reached a major milestone in February with the completion of recruitment for SOFIE’s [18F]FAPI-74 Phase 2 clinical trials. We are currently performing data analysis and behind the scenes planning for Phase 3 is well underway.
On Wednesday, May 14, I will be speaking at PEGS Boston on “[18F]FAPI-74 PET in Gastrointestinal Cancers – Addressing an Unmet Need”. I hope to see you there in person or virtually.
Please share your feedback on how this newsletter can better serve you. We want to hear from you!

Head-to-head comparison of 18F-FDG and 68Ga-FAPI PET/CT in common gynecological malignancies
Cancer Imaging. 2025 Feb 28
FAPI OUTREACH PROGRAM
Join us in advancing the frontier of molecular imaging through the FAPI Outreach Program
SOFIE is continuing to accept applications for our FAPI Outreach Program. This program aims to raise awareness and foster collaboration in the field of molecular imaging, specifically focusing on Fibroblast Activation Protein Inhibitor (FAPI) studies. This program seeks to engage with healthcare professionals, researchers, and the broader community to share knowledge, advancements, and the potential impact of FAPI in diagnostic and therapeutic applications.
*GE Healthcare licensed products and regions are accepting new applications.