
A WORD FROM OUR CHIEF SCIENTIFIC OFFICER
We are excited to launch our FAPI newsletter with the aim of keeping our FAPI user community informed, engage new users and create a community to share research and development advances in FAP targeting efforts. This quarterly newsletter will feature FAPI publication highlights, updates on SOFIE’s FAPI clinical and regulatory development efforts, the latest from our FAPI global outreach program, and any other important news.
In this newsletter we are highlighting the first patient imaged as part of our [68Ga]FAPI-46 Phase 2 Pancreatic Ductal Adenocarcinoma study at NYU Langone, in addition to our IND filing for [18F]FAPI-74 for a Phase 2 study in Gastrointestinal Cancers.
At SOFIE we look forward to expanding our partnership with academic leaders to explore the full potential of the FAPI family of compounds, along with partnerships with industry FAPI-46/FAPI-74 serving as a companion diagnostic tool as a predictive biomarker and therapy response measurement in FAP or stroma targeted therapeutics.
We hope you enjoy this newsletter, and welcome your feedback on how we can better keep you informed.

SOFIE HIGHLIGHTS
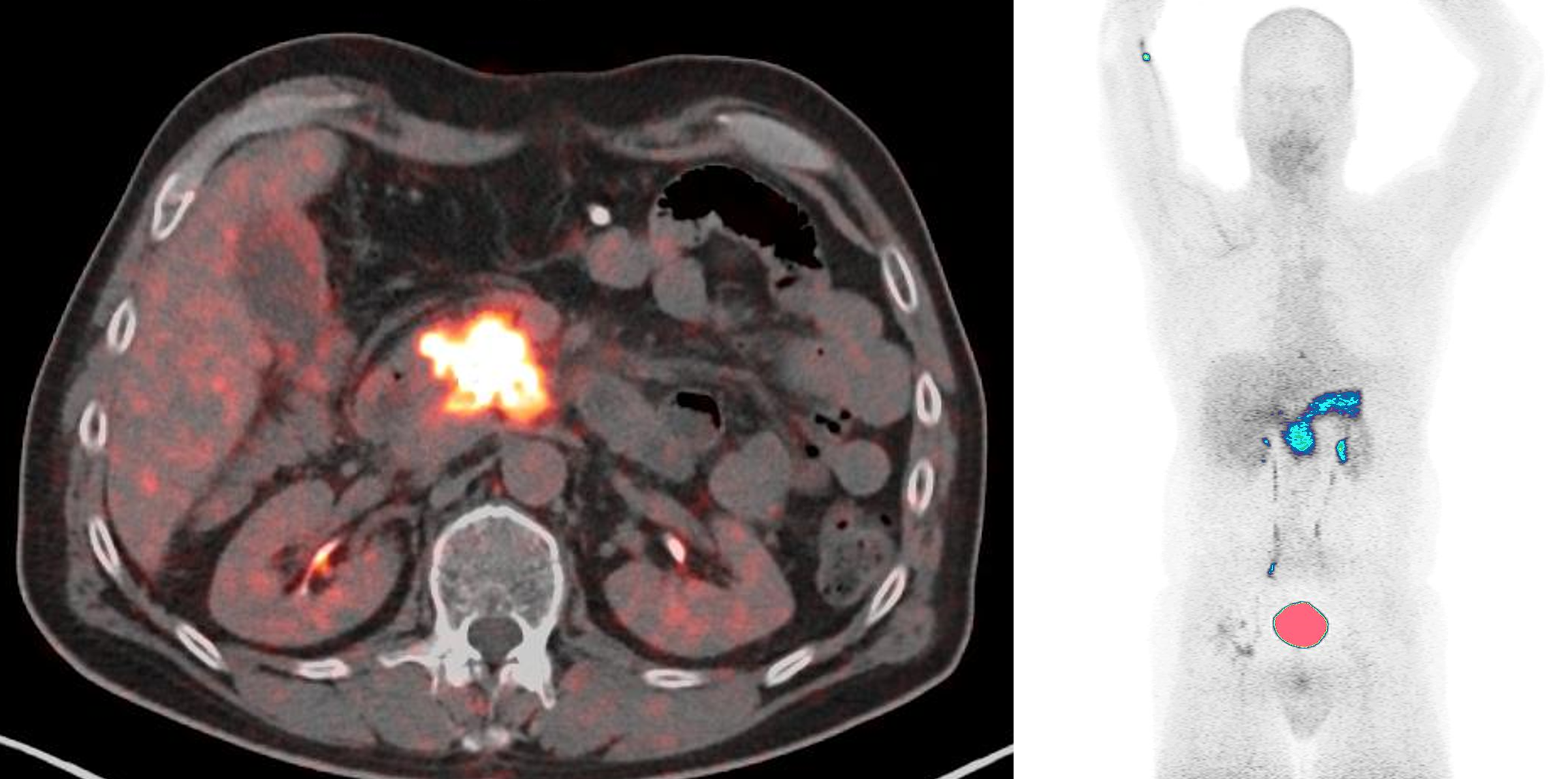
The first patient has been dosed and imaged with [68Ga]FAPI-46 at NYU Langone in SOFIE’s Phase 2, Multicenter, Non-randomized Study of [68Ga]FAPI-46 PET for imaging patients with Pancreatic Ductal Adenocarcinoma (PDAC).
NYU Langone and Mayo Clinic are the first two sites activated in support of this study, with additional sites expected to join in the coming months. Special thanks to NYU Langone Health (PI: Elcin Zan, MD) and Mayo Clinic’s clinical trial team (PI: Ajit H. Goenka, MD) and investigators in support of this study.
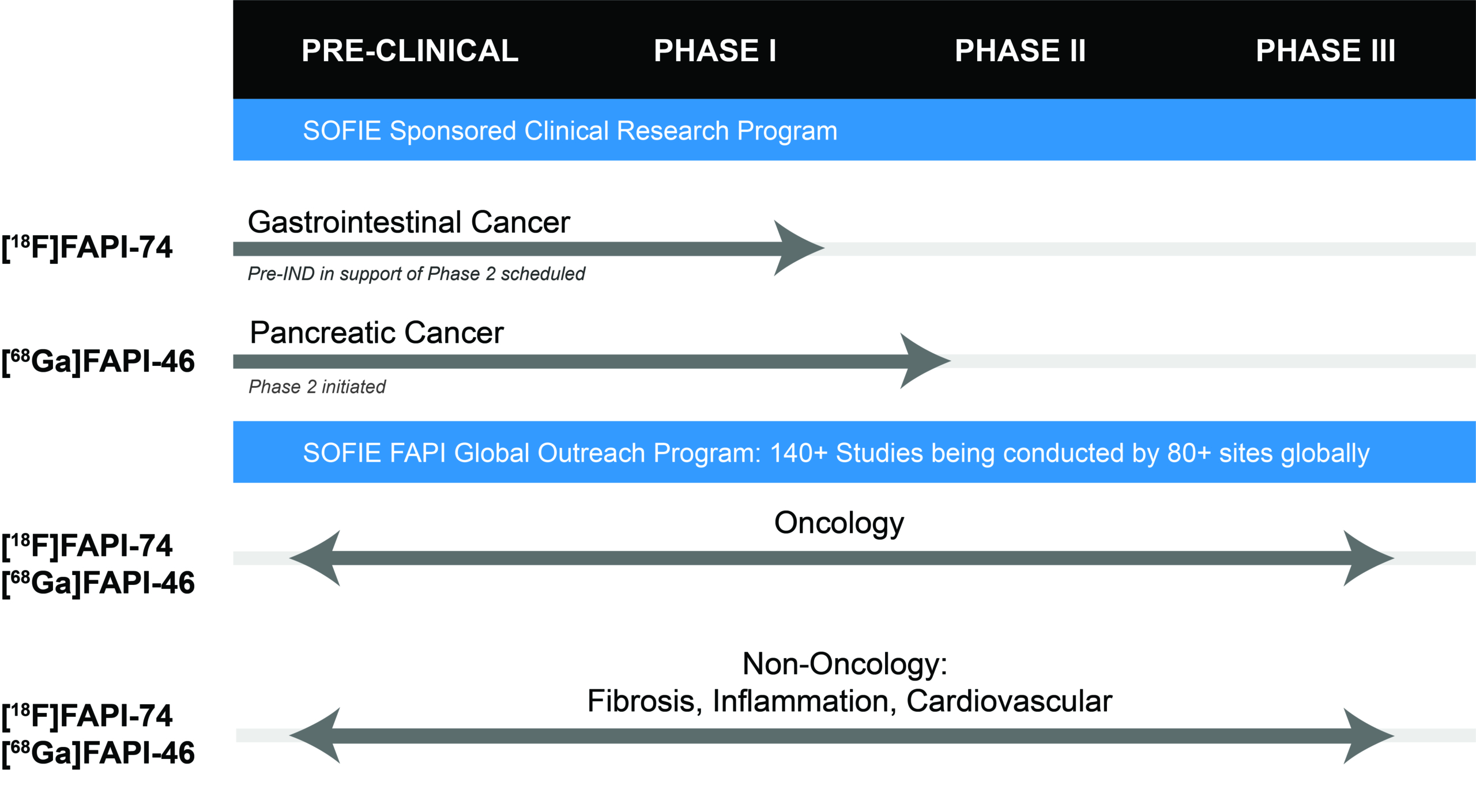
In addition to the efforts on the [68Ga]FAPI-46 PET clinical development, SOFIE has submitted its IND for [18F]FAPI-74 for a Phase 2 study in Gastrointestinal Cancers at the end of October. This submission comes following a successful pre-IND meeting with the FDA in support of this study.
SOFIE is currently in the process of performing site feasibility assessment of clinical trial sites for this study. Once the IND has been granted, SOFIE will move forward with activating clinical trial sites, in addition to working with investigators who are interested in performing investigator initiated studies with [18F]FAPI-74 and providing access to use of the IND through a letter of cross reference.
THIS WEEKEND — DON’T MISS THE ALL-VIRTUAL FAP SUMMIT NOV 4-5!

It’s not too late to register!
In this inaugural event, the ICPO Foundation has assembled a world-class scientific program and invited representatives from academia and industry to dive deep into FAP, its history and role in biology of disease, current research and future applications.
Speakers and moderators include:
- Frederik Giesel, MD (Germany), Scientific Chair
- Uwe Haberkorn, PhD (Germany)
- Rodney Hicks, MD (Australia)
- Ellen Puré, PhD (United States)
Click here to learn more.
FAPI CLINICAL DEVELOPMENT HIGHLIGHTS
PUBLICATION HIGHLIGHTS
Each newsletter will feature three recent FAPI publications.
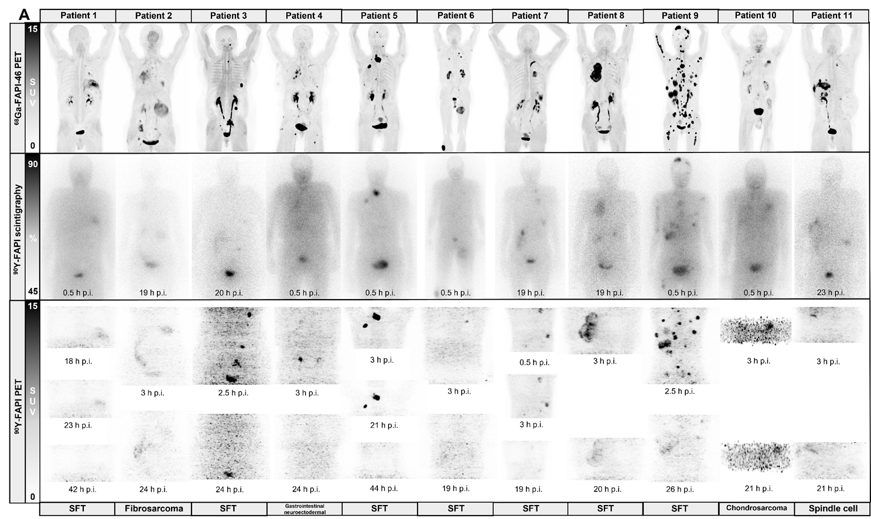
Safety and efficacy of 90Y-FAPI-46 radioligand therapy in patients with advanced sarcoma and other cancer entities
Clin Cancer Res. 2022 Jul 14
![Automated synthesis of [68Ga]Ga-FAPI-46 without pre-purification of the generator eluate on three common synthesis modules and two generator types Automated synthesis of [68Ga]Ga-FAPI-46 without pre-purification of the generator eluate on three common synthesis modules and two generator types](https://sofie.com/wp-content/uploads/2022/09/image-1.png)
![Monitoring Therapeutic Response to Anti-Fibroblast Activation Protein (FAP) CAR T Cells using [18F]AlF-FAPI-74 Monitoring Therapeutic Response to Anti-Fibroblast Activation Protein (FAP) CAR T Cells using [18F]AlF-FAPI-74](https://sofie.com/wp-content/uploads/2022/09/image-3.png)
Monitoring Therapeutic Response to Anti-Fibroblast Activation Protein (FAP) CAR T Cells using [18F]AlF-FAPI-74
Clin Cancer Res. 2022 Aug 16





