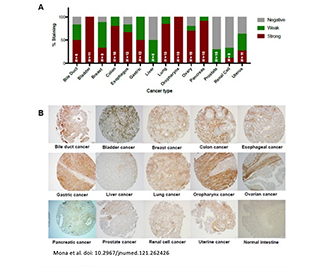
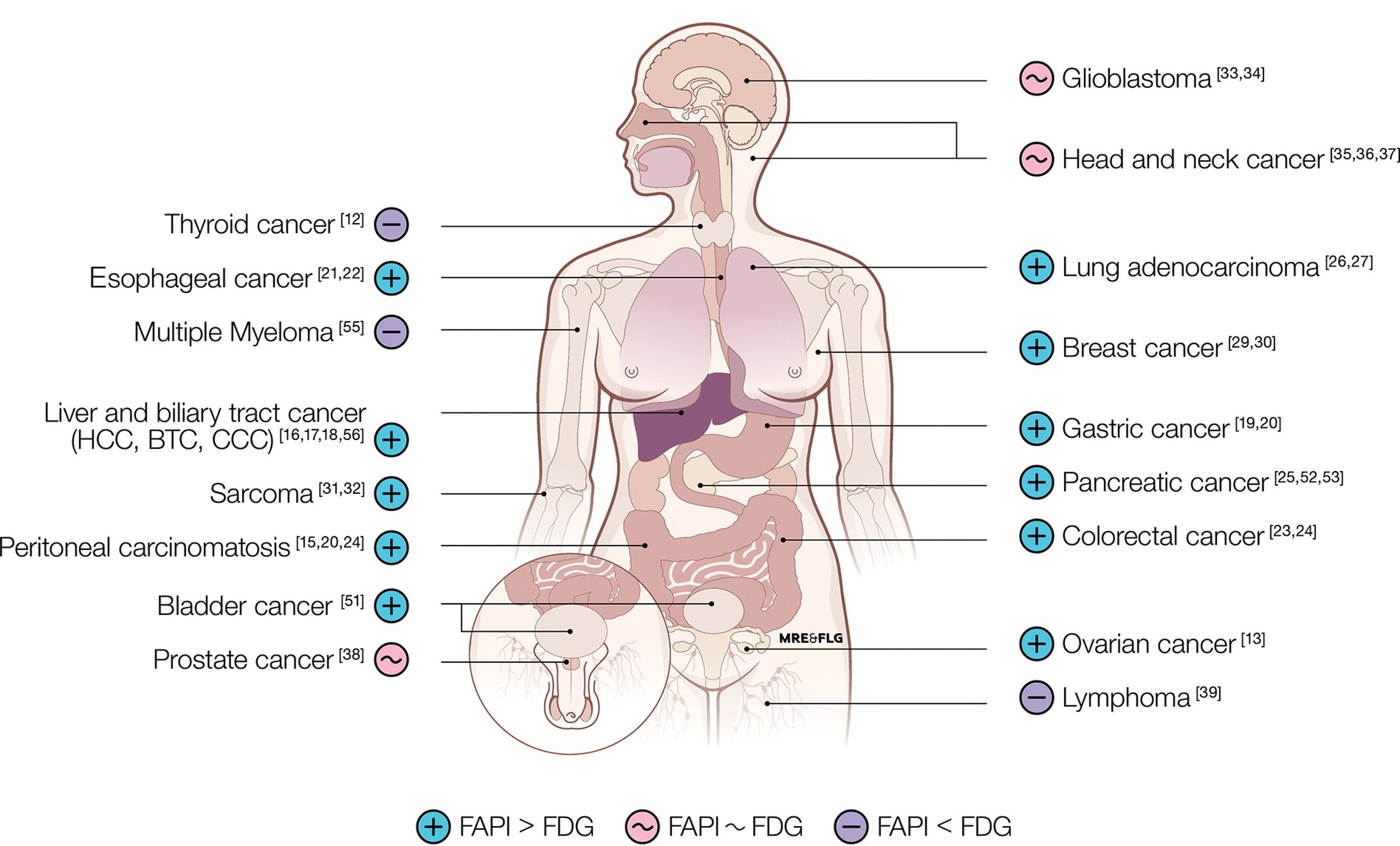
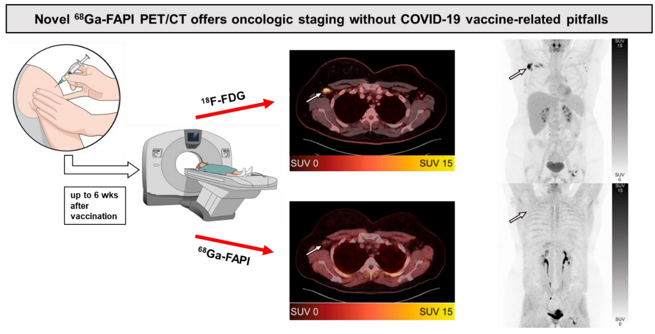
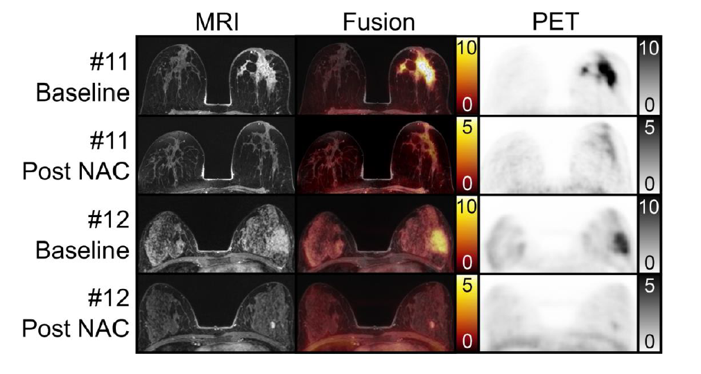
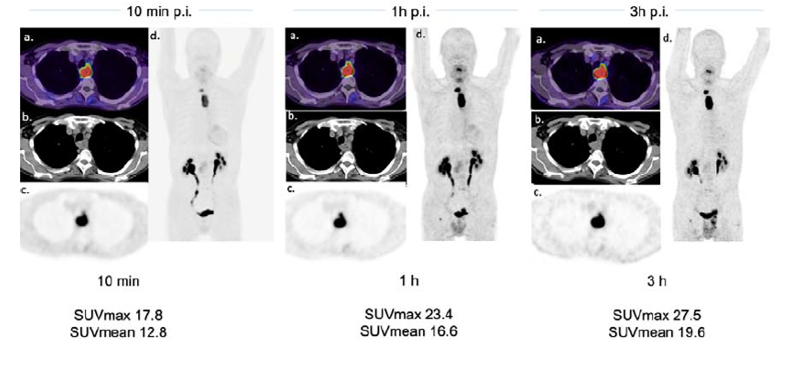
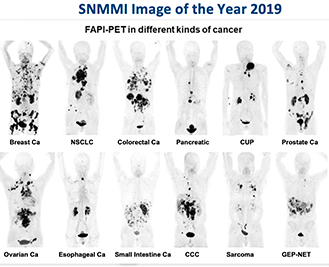
Fibroblast Activation Protein (FAP) Inhibitors as Biomarkers in Oncology, Fibrotic Disease, and Cardiovascular Disease
Presented by: Sherly Mosessian, PhD, SOFIE Chief Scientific Officer
Informa Connect: Radiopharmaceuticals and Imaging Digital Conference
March […]