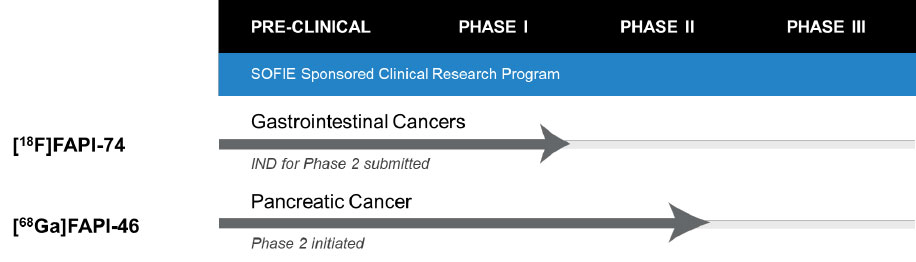
Dulles, VA – October 31, 2022 – SOFIE Biosciences (SOFIE), an established US manufacturer and developer of radiopharmaceuticals, has submitted the IND for [18F]FAPI-74, fluorine 18 labelled radiopharmaceutical targeting Fibroblast Activation Protein (FAP) to the FDA. The clinical protocol for this IND is a Phase 2, Multicenter, Single Arm, Open Label, Non-Randomized Study of [18F]FAPI-74 PET in Patients with Gastrointestinal Cancers.
The filing, which continues SOFIE’s FAPI clinical development efforts, follows the IND activation and clinical trial launch for [68Ga]FAPI-46, the lead 68Ga labelled product of the FAPI family of compounds.
Sherly Mosessian, Ph.D, SOFIE’s Chief Scientific Officer, stated, “We are proud of the work that has gone into enabling this critical milestone for a new and much needed 18F labelled radiopharmaceutical for targeting FAP. This allows us to address an unmet diagnostic need in gastrointestinal cancers, in addition to providing a strong companion diagnostic for radioligand and non-radioligand therapies targeting FAP and the tumor microenvironment.”
While the FDA conducts its 30-day IND review, SOFIE is engaging in site feasibility and vendor selection efforts in preparation for clinical trial activation.