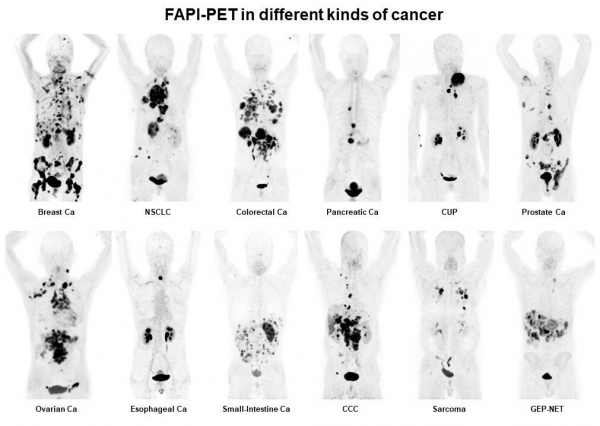
FAPI DEVELOPMENT
FAPI
SOFIE licensed a family of theranostic compounds that target the tumor stroma from the University of Heidelberg, the team that invented PSMA-617 (Novartis). The FAPI compounds inhibit the fibroblast activation protein, which is an important cancer target. Over 500 patients have been imaged to date with the diagnostic compound and initial therapeutic studies are underway. FAPI received the Product/Image/Publication of the Year Award at the Society for Nuclear Medicine and Molecular Imaging.
“FAPI is opening new doors in nuclear medicine, not only in imaging but also in theranostics.”
Dr. Frederick Giesel, M.D, M.B.A.
Universität Heidelberg · Nuclear Medicine
PUBLICATIONS
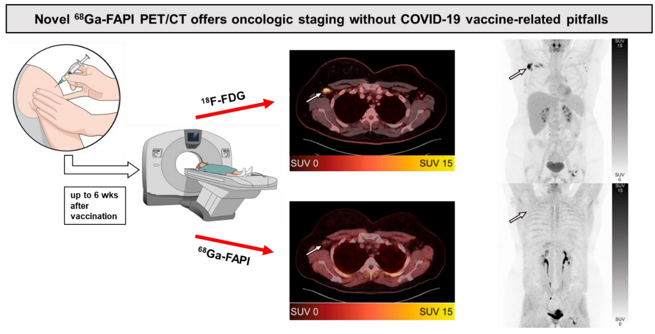
Novel 68Ga-FAPI PET/CT offers oncologic staging without COVID-19 vaccine-related pitfalls
Tristan T Demmert 1, Ines Maric 1, Kelsey L Pomykala 2, Katharina Lueckerath 1, Jens Siveke 3, Benedikt M Schaarschmidt 4, Rainer Hamacher 3, Ken Herrmann 1, Wolfgang P Fendler 1 Abstract In the setting of ongoing SARS-CoV-2 vaccination, vaccine-related tracer uptake in locoregional lymph nodes has become a well-known issue in tumor staging by 18F-FDG PET/CT. 68Ga-FAPI PET/CT is a new oncologic [...]
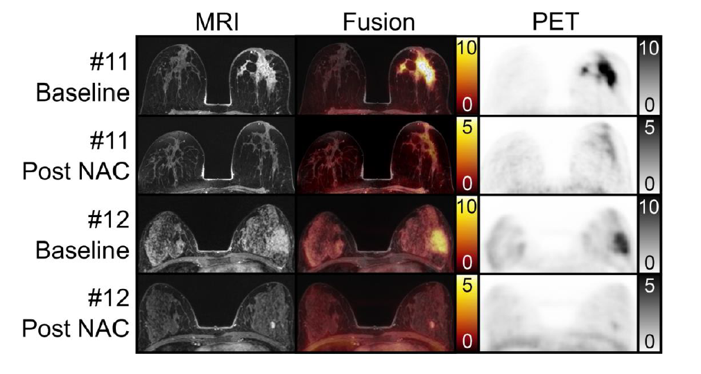
Initial Results of FAPI-PET/MRI to Assess Response to Neoadjuvant Chemotherapy in Breast Cancer
Philipp Backhaus 1, Matthias C Burg 2, Inga Asmus 1, Michaela Pixberg 1, Florian Büther 1, Hans-Jörg Breyholz 1, Randy Yeh 3, Stefanie B Weigel 2, Patricia Stichling 2, Walter Heindel 2, Stefanie Bobe 4, Peter Barth 5, Joke Tio 6, Michael Schäfers 1 Abstract Rationale: Improving imaging-based response following neoadjuvant chemotherapy (NAC) in breast cancer assessment could obviate the need for histologic confirmation of pathological complete response (pCR) and facilitate de-escalation [...]
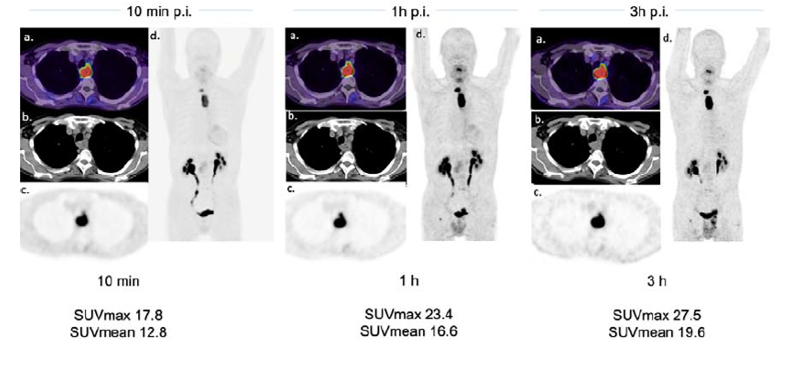
3-time-point PET-analysis of FAPI-46 in a variety of cancers
Mahnoosh Naeimi 1, Peter L Choyke 2, Katharina Dendl 1, Yuriko Mori 3, Fabian Staudinger 1, Tadashi Watabe 4, Stefan A Koerber 5, Manuel Röhrich 1, Juergen Debus 5, Clemens Kratochwil 1, Uwe A Haberkorn 1, Frederik L Giesel 1 Abstract Purpose: A growing family of 68Ga-FAPI Positron Emission Tomography (PET) probes have shown promise in imaging a variety of medical conditions. 68Ga-FAPI-46 in particular has emerged as unique for [...]
INTERESTED IN FAPI?
Guided by our Scientific Advisory Board, sanctioned peer-reviewed publications, and our own FAPI development plan, we remain committed to the process of making FAPI precursor material and radiolabeled compounds readily available.

HOSPITALS
Are you a hospital or clinic interested in participating in our clinical trial? Following the filing of its IND, we will be launching a FAPI-based clinical trial. We expect to collaborate to achieve the required designed endpoints.

RESEARCHERS
Are you an academic researcher interested in evaluating the FAPI compounds within the preclinical and clinical space for both mainstream and unique applications? Our partners at ABX will have you up and running quickly.

PHARMACEUTICAL COMPANIES
Are you a pharmaceutical company or distribution network interested obtaining FAPI for your projects and patients? We are eager to learn more about how our compounds can facilitate your needs. Contact us today.
COMMERCIAL PARTNERS

SOFIE has selected an automated radiosynthesizer for standardized production of its Ga-68 FAPI and F-18 FAPI. This will facilitate production at SOFIE’s facilities for supply to its clinical trial sites.

US-based FAPI-46 and FAPI-74 Tox studies have been completed by Covance at its Indianapolis facility.

ABX is SOFIE’s partner of choice for our FAPI precursor production. ABX has successfully completed the manufacture and shipment of the first stocks of FAPI precursor material, enabling our distribution of precursor and reference standards under MTAs to multiple approved users in the US and EU.
FAPI is not currently approved for general clinical use and should therefore be utilized in accordance with established regulatory guidelines in each jurisdiction.
SCIENTIFIC ADVISORY BOARD

Uwe Haberkorn, MD, PhD
-
Inventor of the FAPI theranostic family
-
Chair of the Department of Nuclear Medicine at the University of Heidelberg
-
Head of the Clinical Cooperation Unit of Nuclear Medicine at the DKFZ

Stefano Fanti, MD
-
Chairman of FAPI Scientific Advisory Board
-
Head of Nuclear Medicine, University of Bologna

Frederick Giesel, MD, MBA
-
Vice Chair of Nuclear Medicine, Department of Nuclear Medicine, University Hospital Heidelberg
-
Full Professor at Osaka University, Osaka Japan and expert in the field oncological theranostics

Clemens Kratochwil, MD
-
Attending and Director, Therapy Unit, Department of Nuclear Medicine at the University of Heidelberg

Jens Siveke, MD
-
Head of the Division of Solid Tumor Translational Oncology (DKFZ/DKTK partner site Essen)
-
Full Professor in the Department of Medical Oncology at the West German Cancer Center, University Hospital Essen

Johannes Czernin, MD
-
Nuclear Medicine and Internal Medicine
-
Chief, Ahmanson Translational Imaging Division at UCLA

Ken Herrmann, MD, MBA
-
Chair of Nuclear Medicine at the University of Duisburg-Essen