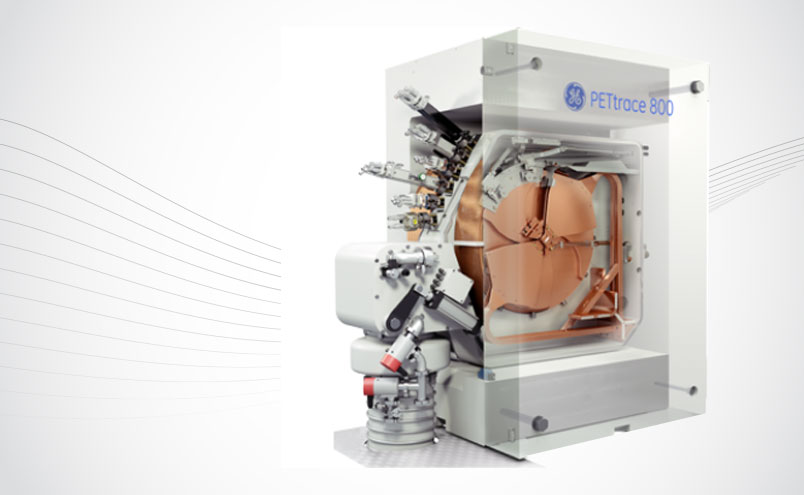
SOFIE has placed a purchase order for GE Healthcare’s top-of-the-line PETtrace 890 dual-particle cyclotron. The machine will be installed in September upon the completion of the current expansion of the company’s PET radiopharmacy in Sterling, VA, making it the 5th dual cyclotron facility in SOFIE’s US Radiopharmacy Network. The addition of the PETtrace 890 will significantly increase its capacity to produce products like FDG and Lantheus’ PyL™ (18F-DCFPyL) to customers in Northern Virginia, Philadelphia, DC, and Maryland.
Nasrin Pourkiani, SOFIE’s Director of Optimization and Sterling Facility Manager said, “It’s exhilarating to observe SOFIE’s Sterling site evolving over the years from manufacturing and dispensing only FDG doses with a single GE cyclotron to now adding specialty products. The growing product line forecasts can be met and exceeded by the addition of this new cyclotron, bringing a much higher capacity to the site. The future is quite promising for the facility, the company, and the products.”
SOFIE’s Director of Technical Services, Brad Knorr, said, “The technology development in cyclotrons over the last five years has produced impressive gains, resulting in increased production and reliability. It was a difficult decision based on the available space, delivery time, and the existence of the facility’s original GE PETtrace cyclotron, but we felt GE was the right choice.”

![SOFIE Biosciences Announces First Patient Dosed in [18F]FAPI-74 Phase 3 Study for Pancreatic Cancer](https://sofie.com/wp-content/uploads/2026/02/FAPIPRO_SOFIE2-500x383.jpg)
![SOFIE drives U.S. manufacture of [18F]FAPI-74](https://sofie.com/wp-content/uploads/2026/01/FAPI-Site-Map-2026-updated-500x383.png)

![SOFIE Biosciences Announces First Patient Dosed in [18F]FAPI-74 Phase 3 Study](https://sofie.com/wp-content/uploads/2025/12/P4_SOFIE_image-for-news-post-on-website-500x383.jpg)
![SOFIE activates FAPI-PRO, second [18F]FAPI-74 Phase 3 trial](https://sofie.com/wp-content/uploads/2025/12/FAPI_SOFIE-500x383.jpg)