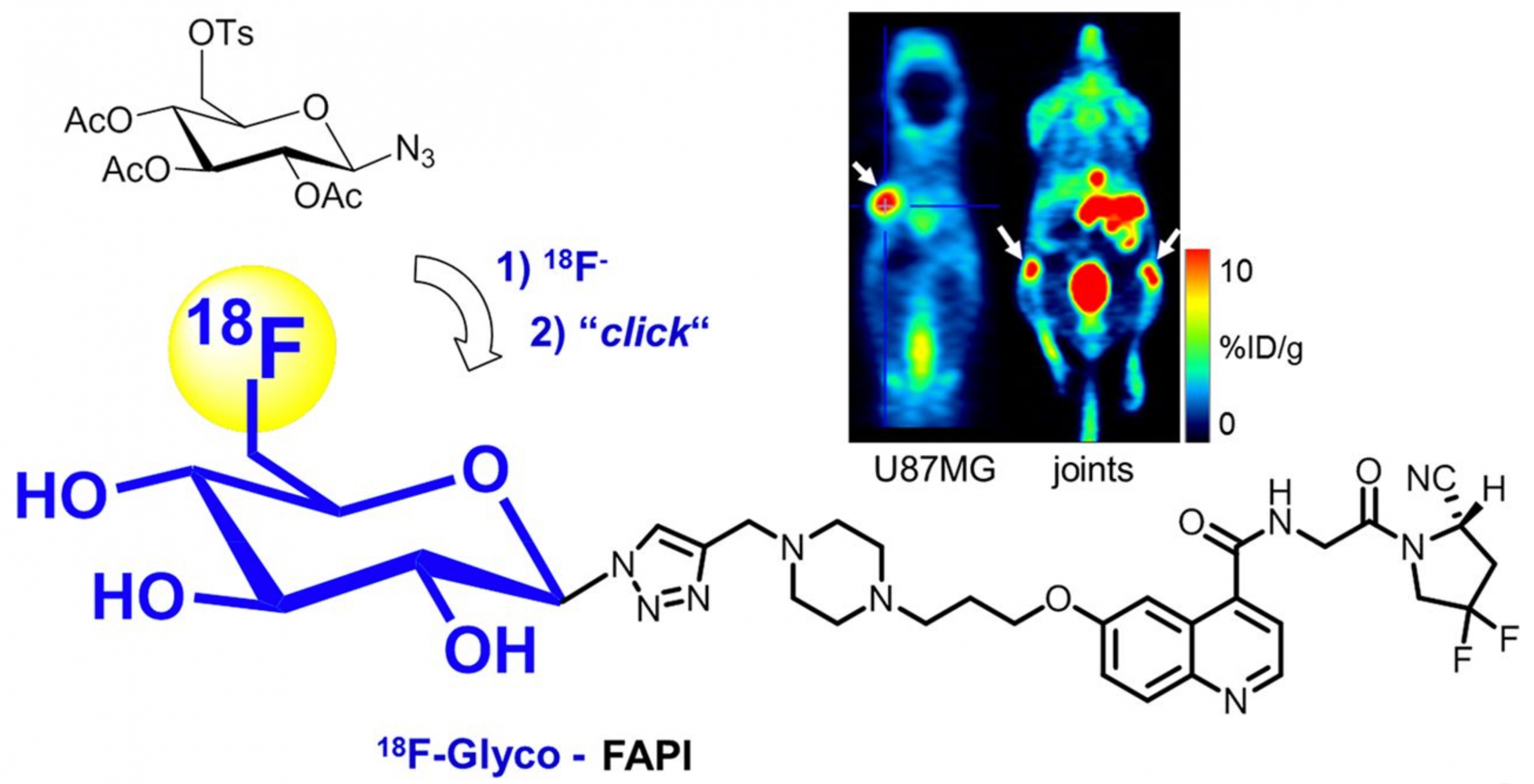
Fibroblast activation protein (FAP) has emerged as an interesting molecular target used in the imaging and therapy of various types of cancers. 68Ga-labeled chelator-linked FAP inhibitors (FAPIs) have been successfully applied to PET imaging of various tumor types. To broaden the spectrum of applicable PET tracers for extended imaging studies of FAP-dependent diseases, we herein report the radiosynthesis and preclinical evaluation of an 18F-labeled glycosylated FAPI ([18F]FGlc-FAPI).
Methods: An alkyne-bearing precursor was synthesized and subjected to click chemistry-based radiosynthesis of [18F]FGlc-FAPI by 2-step 18F-fluoroglycosylation. FAP-expressing HT1080hFAP cells were used to study competitive binding to FAP, cellular uptake, internalization, and efflux of [18F]FGlc-FAPI in vitro. Biodistribution studies and in vivo small-animal PET studies of [18F]FGlc-FAPI compared with [68Ga]Ga-FAPI-04 were conducted in nude mice bearing HT1080hFAP tumors or U87MG xenografts.
Results: [18F]FGlc-FAPI was synthesized with a 15% radioactivity yield and a high radiochemical purity of more than 99%. In HT1080hFAP cells, [18F]FGlc-FAPI showed specific uptake, a high internalized fraction, and low cellular efflux. Compared with FAPI-04 (half maximal inhibitory concentration [IC50] = 32 nM), the glycoconjugate, FGlc-FAPI (IC50 = 167 nM), showed slightly lower affinity for FAP in vitro, whereas plasma protein binding was higher for [18F]FGlc-FAPI. Biodistribution studies revealed significant hepatobiliary excretion of [18F]FGlc-FAPI; however, small-animal PET studies in HT1080hFAP xenografts showed higher specific tumor uptake of [18F]FGlc-FAPI (4.5 percentage injected dose per gram of tissue [%ID/g]) than of [68Ga]Ga-FAPI-04 (2 %ID/g). In U87MG tumor-bearing mice, both tracers showed similar tumor uptake, but [18F]FGlc-FAPI showed a higher tumor retention. Interestingly, [18F]FGlc-FAPI demonstrated high specific uptake in bone structures and joints.
Conclusion: [18F]FGlc-FAPI is an interesting candidate for translation to the clinic, taking advantage of the longer half-life and physical imaging properties of 18F. The availability of [18F]FGlc-FAPI may allow extended PET studies of FAP-related diseases, such as cancer, but also arthritis, heart diseases, or pulmonary fibrosis.
Affiliations:
- Friedrich-Alexander University Erlangen-Nürnberg (FAU), Department of Nuclear Medicine, Molecular Imaging and Radiochemistry, Translational Research Center, Erlangen, Germany.
- Friedrich-Alexander University Erlangen-Nürnberg (FAU), Department of Nephropathology, Erlangen, Germany; and.
- Friedrich-Alexander University Erlangen-Nürnberg (FAU), Department of Nuclear Medicine, Erlangen, Germany.
- Friedrich-Alexander University Erlangen-Nürnberg (FAU), Department of Nuclear Medicine, Molecular Imaging and Radiochemistry, Translational Research Center, Erlangen, Germany olaf.prante@uk-erlangen.de.

![Efficacy of [68Ga]Ga-FAPI-PET as a non-invasive evaluation method of liver fibrosis](https://sofie.com/wp-content/uploads/2025/06/info.ibamolecular-500x383.png)
![Comparison of [99mTc]Tc-FAPI SPECT/CT and [18F]FDG PET/CT as predictive biomarkers for immunotherapy response in gastrointestinal cancer](https://sofie.com/wp-content/uploads/2025/06/info.ibamolecular-500x383.jpg)