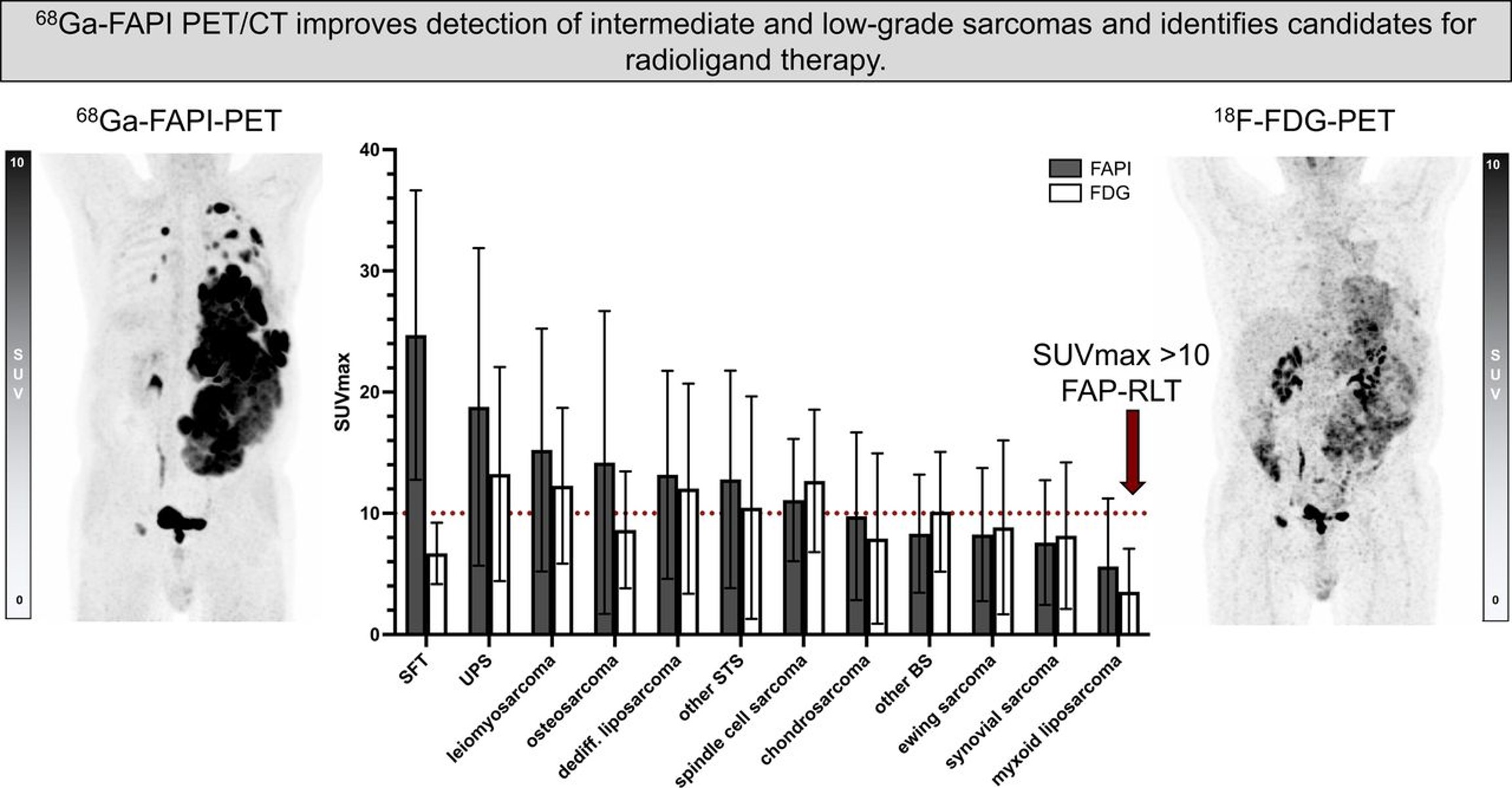
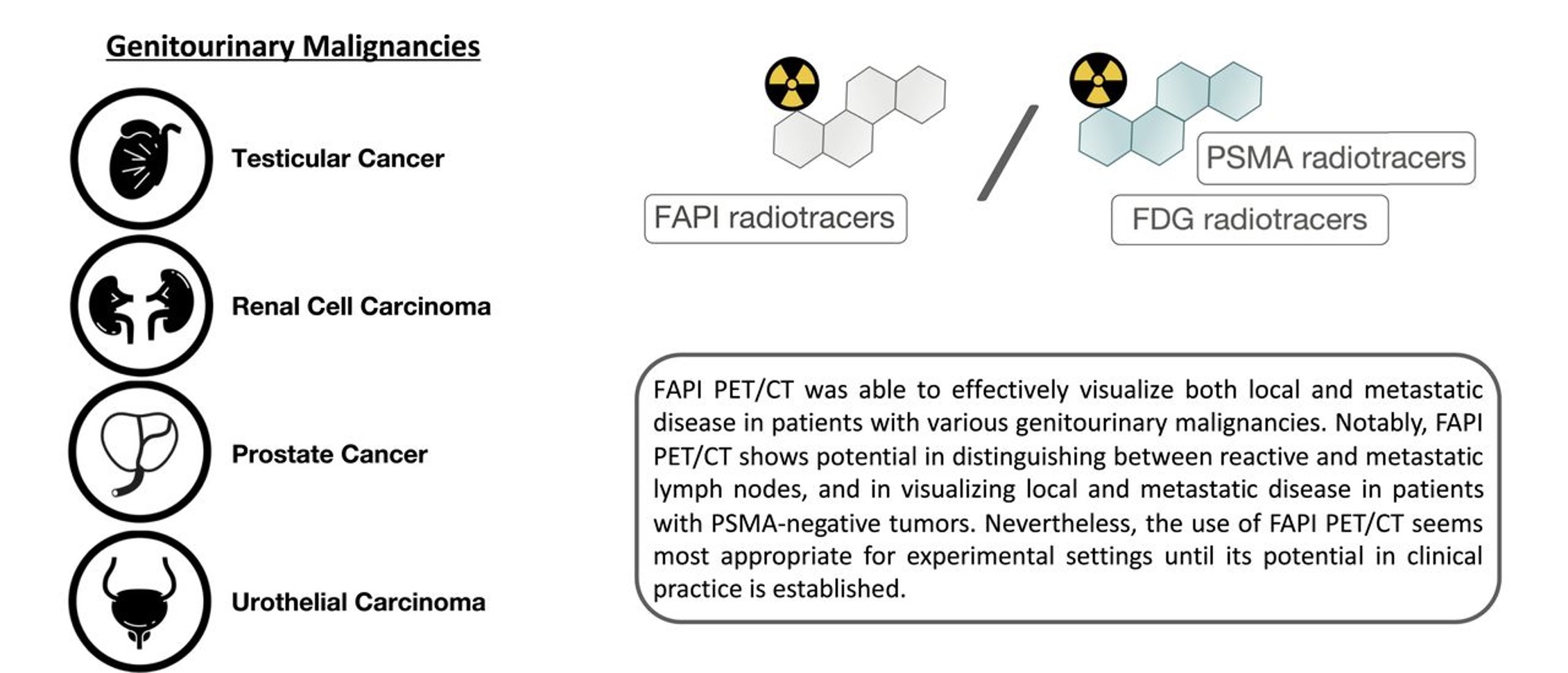
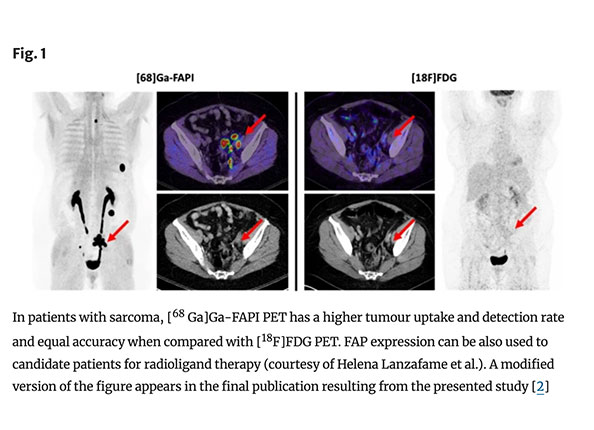
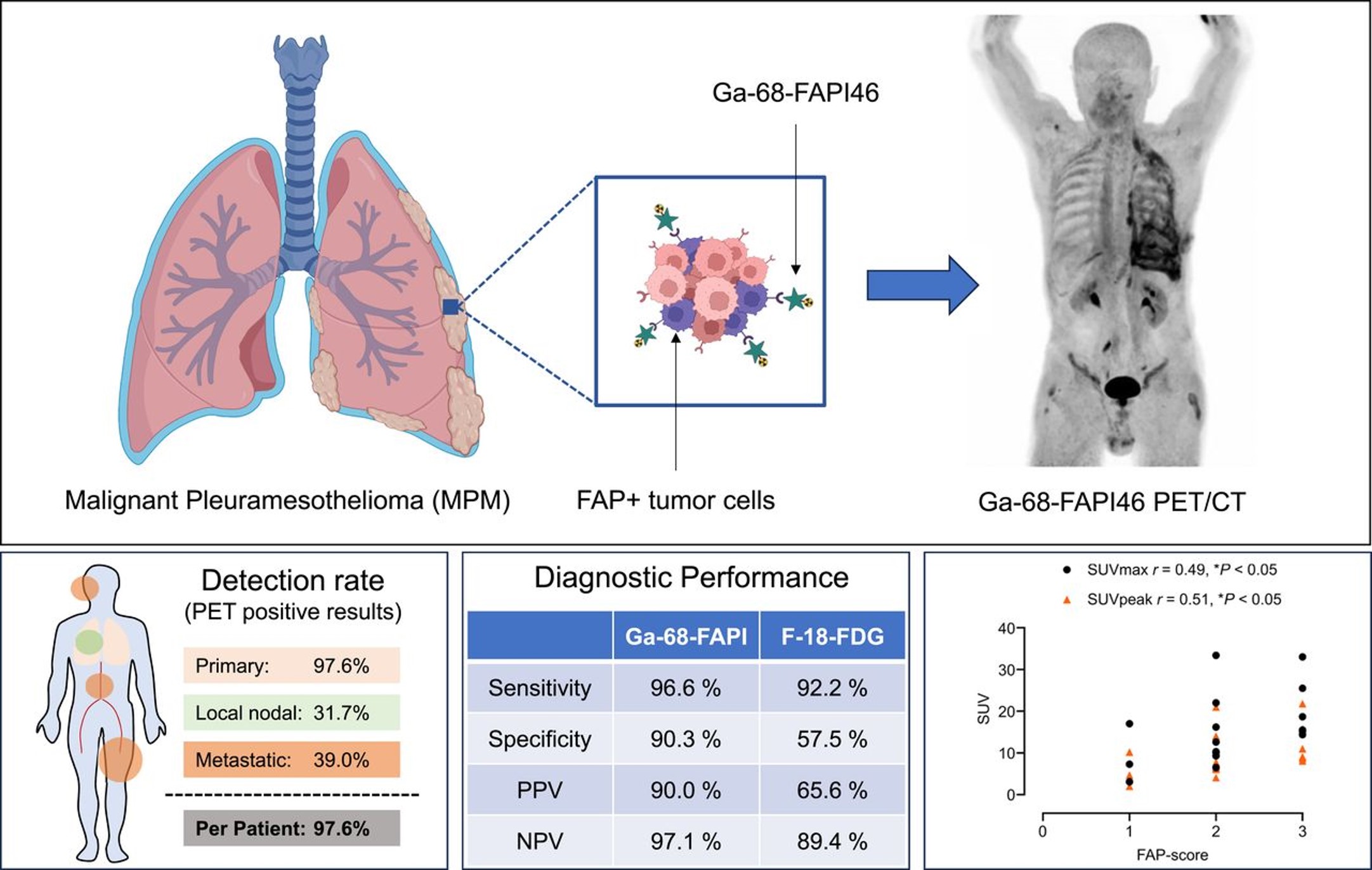
Fibroblast Activation Protein-Directed Imaging Outperforms 18F-FDG PET/CT in Malignant Mesothelioma: A Prospective, Single-Center, Observational Trial
Lukas Kessler, Felix Schwaning, Martin Metzenmacher, Kim Pabst, Jens Siveke, Marija Trajkovic-Arsic, Benedikt Schaarschmidt, Marcel […]


![[68Ga]Ga-FAPI-46 PET/CT for penile cancer - a feasibility study](https://sofie.com/wp-content/uploads/2024/06/info.ibamolecular.png)