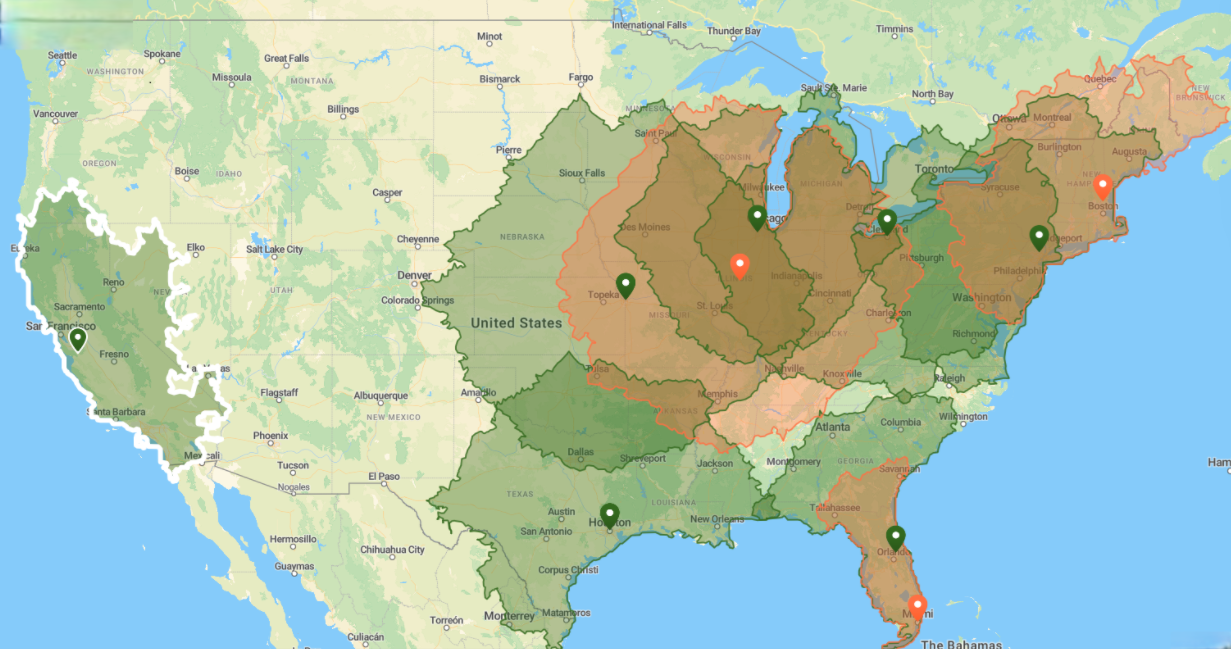
Seven SOFIE radiopharmacies now produce the FAP-targeting radiopharmaceutical, as SOFIE launches Phase 3 clinical trials and support of Pharma and Academic groups. Map shows planned [18F]FAPI-74 U.S. Coverage for 2026.
Kansas City, MO, and Houston, TX, are the latest SOFIE radiopharmacies cleared to manufacture [18F]FAPI-74, bringing the total number of sites to seven. The other locations producing [18F]FAPI-74 are Totowa, NJ; Gilroy, CA; Romeoville, IL; Cleveland, OH; and Sanford, FL. This expansion of FAPI-74 manufacturing capability is well-timed coinciding with launch of SOFIE’s Phase 3 studies: FAPI-GO (NCT07217704) and FAPI PRO (NCT07217717).
SOFIE’s FAPI-74 production power further enables SOFIE to develop companion use of [18F]FAPI-74 in both Radioligand Therapy (RLT) and non-RLT Pharma collaborations. For example, SOFIE is a supplier of [18F]FAPI-74 for Lilly’s FiREBOLT FAP Radioligand Therapy study (NCT07213791).
“SOFIE is proud to have continued to broaden the reach of [18F]FAPI-74 to our Kansas City, MO and Houston, TX facilities this year”, said Alyse Zeller, Director of CMC. “We will continue to expand in 2026 to give the best access to patients, their physicians, and sponsors. Together, we hope to forge a path forward with radiotheranostics and [18F]FAPI-74. It’s a testament to our dedicated and knowledgeable Training, Radiochemical Manufacturing, and on-site teams that these efforts are carried through and thriving. We are especially grateful for their hard work and expertise.”
Sangeeta Kalra, Director, FAPI Global Outreach Program, commented, “We’re excited to support a Phase I radiotheranostic trial in which [18F]FAPI-74 will be used as the radiodiagnostic agent. This collaboration marks an exciting step forward in advancing precision oncology, and we look forward to sharing the innovative work ahead.”
Under Zeller’s guidance, SOFIE has completed validation efforts to extend the shelf-life and delivery radius of [18F]FAPI-74 in time for the new year. In 2026, approved delivery sites within the radius of eight hours of activated SOFIE sites will be able to receive doses of the product with an expected 12-hour shelf-life post-End Of Synthesis.
Thank you to our team’s commitment towards successful tech transfer! If you are interested in partnering with SOFIE for access to [18F]FAPI-74, please contact fapiprogram@sofie.com .

![SOFIE Biosciences Announces First Patient Dosed in [18F]FAPI-74 Phase 3 Study for Pancreatic Cancer](https://sofie.com/wp-content/uploads/2026/02/FAPIPRO_SOFIE2-500x383.jpg)

![SOFIE Biosciences Announces First Patient Dosed in [18F]FAPI-74 Phase 3 Study](https://sofie.com/wp-content/uploads/2025/12/P4_SOFIE_image-for-news-post-on-website-500x383.jpg)
![SOFIE activates FAPI-PRO, second [18F]FAPI-74 Phase 3 trial](https://sofie.com/wp-content/uploads/2025/12/FAPI_SOFIE-500x383.jpg)
![SOFIE activates FAPI-GO, first [18F]FAPI-74 Phase 3 trial](https://sofie.com/wp-content/uploads/2025/11/SOFIE_FAPI2-500x383.jpg)