Wolfgang P Fendler, Kim M Pabst, Lukas Kessler, Pedro Fragoso Costa, Justin Ferdinandus, Manuel Weber, Maria Lippert, Katharina Lueckerath, Lale Umutlu, Karina Kostbade, Ilektra A Mavroeidi, Martin Schuler, Marit Ahrens, Christoph Rischpler, Sebastian Bauer, Ken Herrmann, Jens T Siveke, Rainer Hamacher
Abstract
Purpose: We report efficacy and safety of 90Y-FAPI-46-RLT in patients with advanced sarcoma, pancreatic cancer (PDAC) and other cancer entities.
Experimental design: Up to four cycles of RLT were offered to patients with (a) progressive metastatic malignancy, (b) exhaustion of approved therapies, and (c) high fibroblast activation protein (FAP) expression, defined as SUVmax≥10 in more than 50% of tumor. Primary endpoint was RECIST response after RLT. Secondary endpoints included PET response (PERCIST), overall survival, dosimetry and safety of FAP-RLT.
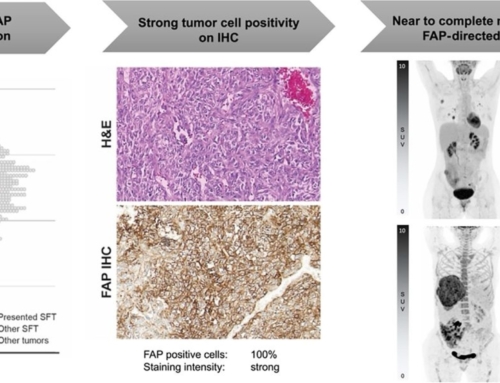
Results: Among n=119 screened patients, n=21 (18%) were found eligible (n=16/3/1/1 sarcoma/PDAC/prostate/gastric cancer; 38% ECOG≥2) and received n=47 90Y-FAPI-46-RLT cycles; n=16/21 (76%) patients underwent repeat RLT. By RECIST disease control was confirmed in n=8/21 patients (38%; 8/16 [50%] of evaluable patients). There were 1 partial response and 7 stable diseases after RLT. Disease control was associated with prolonged overall survival (p=0.013). PERCIST response was noted in n=8/21 patients (38%; 8/15 [53%] of evaluable patients). Dosimetry was acquired in n=19 (90%) patients. Mean absorbed dose was 0.53Gy/GBq in kidney, 0.04Gy/GBq in bone marrow and <0.14Gy/GBq in liver and lung. Treatment-related grade 3 or 4 adverse events were observed in n=8 (38%) patients with thrombocytopenia (n=6) and anemia (n=6) being most prevalent.
Conclusion: 90Y-FAPI-46-RLT was safe and led to RECIST partial response in one case as well as stable disease in about one third of patients with initially progressive sarcomas, PDAC and other cancers. Discontinuation after the first cycle and a low rate of partial response require for future improvement of FAP-RLT.

![FAP Expression in Renal Tumors Assessed by [68Ga]Ga-FAPI-46 PET Imaging and FAP Immunohistochemistry: A Case Series of Six Patients](https://sofie.com/wp-content/uploads/2025/12/info.ibamolecular-scaled-500x383.jpg)
![[68Ga]Ga-API-46 PET accuracy for cancer imaging with histopathology validation: a single-centre, single-arm, interventional, phase 2 trial](https://sofie.com/wp-content/uploads/2025/09/image-500x383.png)