Objectives: To date, there is no valuable tool to assess fibrotic disease activity in humans in vivo in a non-invasive way. This study aims to uncouple inflammatory from fibrotic disease activity in fibroinflammatory diseases such as IgG4-related disease.
Methods: In this cross-sectional clinical study, 27 patients with inflammatory, fibrotic and overlapping manifestations of IgG4-related disease underwent positron emission tomography (PET) scanning with tracers specific for fibroblast activation protein (FAP; 68Ga-FAP inhibitor (FAPI)-04), 18F-fluorodeoxyglucose (FDG), MRI and histopathological assessment. In a longitudinal approach, 18F-FDG and 68Ga-FAPI-04 PET/CT data were evaluated before and after immunosuppressive treatment and correlated to clinical and MRI data.
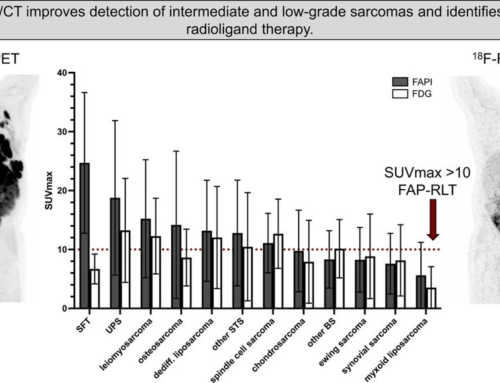
Results: Using combination of 68Ga-FAPI-04 and 18F-FDG-PET, we demonstrate that non-invasive functional tracking of IgG4-related disease evolution from inflammatory towards a fibrotic outcome becomes feasible. 18F-FDG-PET positive lesions showed dense lymphoplasmacytic infiltration of IgG4 + cells in histology, while 68Ga-FAPI-04 PET positive lesions showed abundant activated fibroblasts expressing FAP according to results from RNA-sequencing of activated fibroblasts. The responsiveness of fibrotic lesions to anti-inflammatory treatment was far less pronounced than that of inflammatory lesions.
Conclusion: FAP-specific PET/CT permits the discrimination between inflammatory and fibrotic activity in IgG4-related disease. This finding may profoundly change the management of certain forms of immune-mediated disease, such as IgG4-related disease, as subtypes dominated by fibrosis may require different approaches to control disease progression, for example, specific antifibrotic agents rather than broad spectrum anti-inflammatory treatments such as glucocorticoids.
Affiliations:
- Department of Nuclear Medicine, Friedrich-Alexander-University (FAU) Erlangen-Nürnberg and University Hospital Erlangen, Erlangen, Germany.
- Department of Internal Medicine 3, Rheumatology & Immunology, Friedrich-Alexander-University (FAU) Erlangen-Nürnberg and University Hospital Erlangen, Erlangen, Germany.
- Massachusetts General Hospital Rheumatology Unit, Harvard Medical School, Boston, Massachusetts, USA.
- Department of Nuclear Medicine, Heidelberg University, Heidelberg, Baden-Württemberg, Germany.
- Institute of Radiology, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Bayern, Germany.
- Institute of Pathology, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Bayern, Germany.
- Department of Internal Medicine 3, Rheumatology & Immunology, Friedrich-Alexander-University (FAU) Erlangen-Nürnberg and University Hospital Erlangen, Erlangen, Germany andreas.ramming@uk-erlangen.de.

![[68Ga]Ga-FAPI-46 PET/CT for penile cancer – a feasibility study](https://sofie.com/wp-content/uploads/2024/06/info.ibamolecular-500x383.png)