Purpose: Cancer-associated fibroblasts (CAFs) expressing fibroblast activation protein (FAP) have been associated with the aggressive nature of head and neck cancers (HNCs). These tumours grow diffusely, leading to extremely challenging differentiation between tumour and healthy tissue. This analysis aims to introduce a novel approach of tumour detection, contouring and targeted radiotherapy of HNCs using visualisation of CAFs: PET-CT with 68Ga-radiolabeled inhibitors of FAP (FAPI).
Methods: FAPI PET-CT was performed without complications prior to radiotherapy in addition to contrast enhanced CT (CE-CT) and MRI on 14 patients with HNC. First, for tissue biodistribution analysis, volumes of interest were defined to quantify SUVmean and SUVmax in tumour and healthy parenchyma. Secondly, using four thresholds of three-, five-, seven- and tenfold increase of FAPI enhancement in the tumour as compared with normal tissue, four different gross tumour volumes (FAPI-GTV) were created automatically. These were compared with GTVs created conventionally with CE-CT and MRI (CT-GTV).
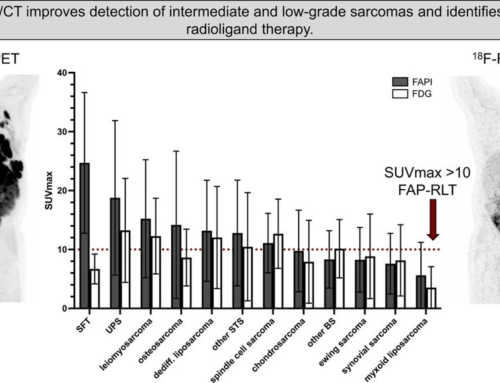
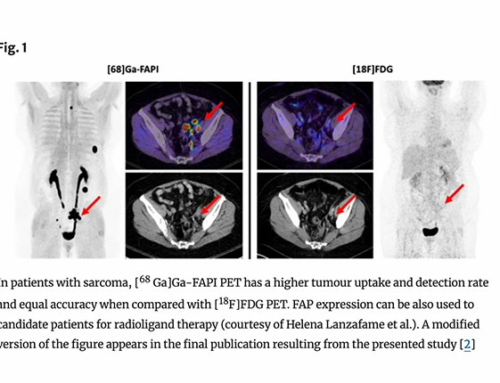
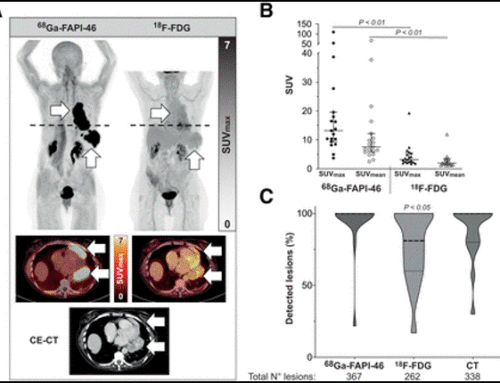
Results: The biodistribution analysis revealed high FAPI avidity within tumorous lesions (e.g. primary tumours, SUVmax 14.62 ± 4.44; SUVmean 7.41 ± 2.39). In contrast, low background uptake was measured in healthy tissues of the head and neck region (e.g. salivary glands: SUVmax 1.76 ± 0.31; SUVmean 1.23 ± 0.28). Considering radiation planning, CT-GTV was of 27.3 ml, whereas contouring with FAPI resulted in significantly different GTVs of 67.7 ml (FAPI × 3, p = 0.0134), 22.1 ml (FAPI × 5, p = 0.0419), 7.6 ml (FAPI × 7, p = 0.0001) and 2.3 ml (FAPI × 10, p = 0.0001). Taking these significant disparities between the GTVs into consideration, we merged FAPI-GTVs with CT-GTVs. This resulted in median volumes, that were, as compared to CT-GTVs, significantly larger with FAPI × 3 (54.7 ml, + 200.5% relative increase, p = 0.0005) and FAPI × 5 (15.0 ml, + 54.9%, p = 0.0122). Furthermore, FAPI-GTVs were not covered by CE-CT-based planning target volumes (CT-PTVs) in several cases.
Conclusion: We present first evidence of diagnostic and therapeutic potential of FAPI ligands in head and neck cancer. Larger studies with histopathological correlation are required to validate our findings.
Affiliations:
- Department of Nuclear Medicine, University Hospital RWTH Aachen University, Pauwelsstr. 30, 52074, Aachen, Germany.
- Center of Integrated Oncology (CIO), Universities of Aachen, Bonn, Cologne, and Duesseldorf, Cologne, Germany.
- Department of Nuclear Medicine, University Hospital RWTH Aachen University, Pauwelsstr. 30, 52074, Aachen, Germany. fmottaghy@ukaachen.de.
- Center of Integrated Oncology (CIO), Universities of Aachen, Bonn, Cologne, and Duesseldorf, Cologne, Germany. fmottaghy@ukaachen.de.
- Department of Radiology and Nuclear Medicine, Maastricht University Medical Center (MUMC+), P. Debeylaan 25, 6229 HX Maastricht, P.O. Box 5800, 6202 AZ, Maastricht, The Netherlands. fmottaghy@ukaachen.de.

![[68Ga]Ga-FAPI-46 PET/CT for penile cancer – a feasibility study](https://sofie.com/wp-content/uploads/2024/06/info.ibamolecular-500x383.png)