RESOURCES
Focus on FAPI Newsletter – Spring 2023
A WORD FROM OUR CHIEF SCIENTIFIC OFFICER Dear FAPI community, This quarter, the FAPI team celebrated several impressive milestones. We activated our [18F]FAPI-74 study in GI Cancers in the US with Massachusetts General Hospital as our first site and the first patient imaged on [...]
SOFIE partners with BAMF Health, diversifying sites for Phase II [68Ga]FAPI-46 study
SOFIE has added BAMF Health, a novel cancer treatment center in Grand Rapids, Michigan, as its latest site in SOFIE’s sponsored clinical trial, “A Phase 2, Multicenter, Single- Arm, Open-Label Non-Randomized Study of [68Ga]FAPI-46 PET in Patients with resectable or borderline resectable Pancreatic Ductal [...]
First Patient Imaged in SOFIE’s 18F-FAPI Phase 2 trial
Dulles, VA –May 12, 2023 – SOFIE Biosciences (SOFIE), an established US manufacturer and developer of radiopharmaceuticals, has dosed and imaged the first patient with [18F]FAPI-74 in its US Clinical Trial of flourine-18 labeled Fibroblast Activation Protein Inhibitor (FAPI). This study is a Phase [...]
FAPI Theragnostics
Presented by: Prof. Dr. Frederik L. Giesel, MD, MBA; Chair of Nuclear Medicine, Department of Nuclear Medicine; University Hospital Düsseldorf, Germany World Theragnostics Day – Focus on FAPI March 31, 2023 Watch Webinar
Fibroblast Activation Protein (FAP) Inhibitors as Biomarkers in Oncology, Fibrotic Disease, and Cardiovascular Disease
Presented by: Sherly Mosessian, PhD, SOFIE Chief Scientific Officer Informa Connect: Radiopharmaceuticals and Imaging Digital Conference March 14, 2023 Download the Presentation
Focus on FAPI Newsletter – Winter 2023
A WORD FROM OUR CHIEF SCIENTIFIC OFFICER Dear FAPI community, We would like to wish you a prosperous year heading into 2023. We ended 2022 with a number of exciting developments on the FAPI front. SOFIE served as the major sponsor for the [...]
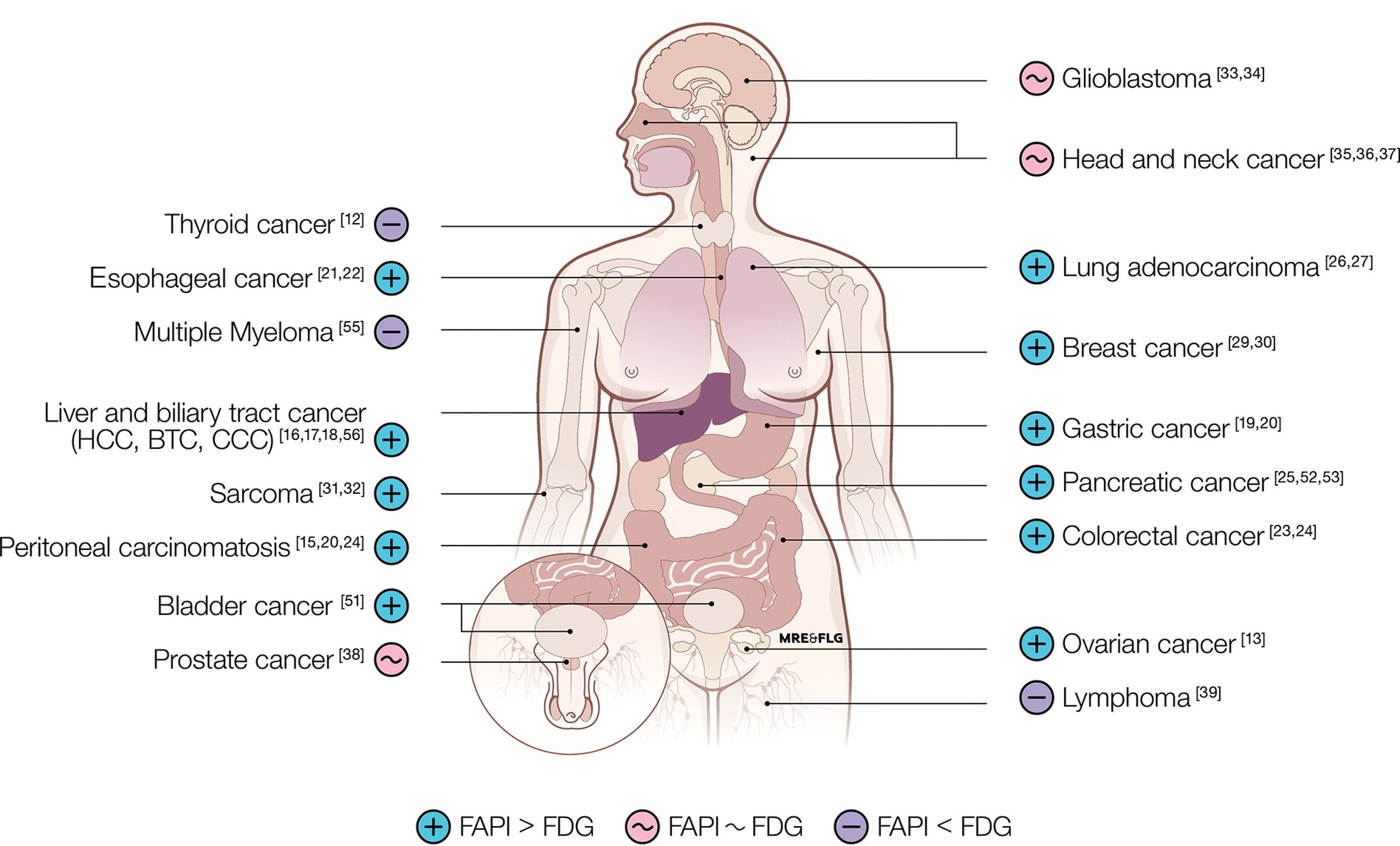
FAPI PET: Fibroblast Activation Protein Inhibitor Use in Oncologic and Nononcologic Disease
Yuriko Mori 1, Katharina Dendl 1, Jens Cardinale 1, Clemens Kratochwil 1, Frederik L Giesel # 1, Uwe Haberkorn # 1 Abstract Gallium 68 (68Ga)-labeled fibroblast activation protein (FAP) inhibitor (FAPI) PET is based on the molecular targeting of the FAP, which is known to be highly expressed in the major cell population in tumor stroma, termed [...]
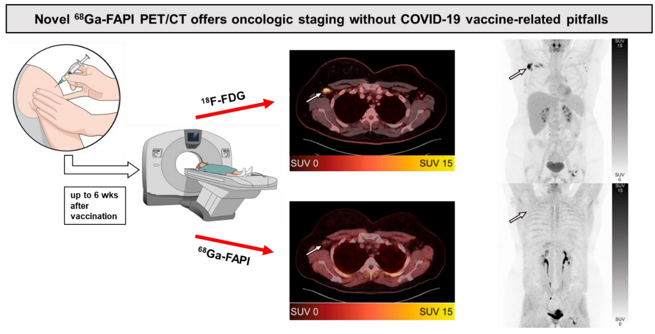
Novel 68Ga-FAPI PET/CT offers oncologic staging without COVID-19 vaccine-related pitfalls
Tristan T Demmert 1, Ines Maric 1, Kelsey L Pomykala 2, Katharina Lueckerath 1, Jens Siveke 3, Benedikt M Schaarschmidt 4, Rainer Hamacher 3, Ken Herrmann 1, Wolfgang P Fendler 1 Abstract In the setting of ongoing SARS-CoV-2 vaccination, vaccine-related tracer uptake in locoregional lymph nodes has become a well-known issue in tumor staging by 18F-FDG PET/CT. 68Ga-FAPI PET/CT is a new oncologic [...]
SOFIE and Mayo Clinic collaborate to secure $3M NIH grant for FAPI clinical trial
The National Cancer Institute (NCI) has awarded Mayo Clinic (Rochester), in partnership with SOFIE, a five-year R01 clinical trial, "Quantitative In Vivo 68Ga-Fibroblast-Activation-Protein-Inhibitors (FAPI)-46 PET Imaging of Cancer-Associated Fibroblasts (CAFs) in Pancreatic Ductal Adenocarcinoma (PDA)." The $3.3 million grant will enable the research team, [...]
IND granted for FAP targeting therapeutic in SOFIE partnership
Dulles, VA – January 17, 2023 – SOFIE Biosciences (SOFIE), an established US manufacturer and developer of radiopharmaceuticals, will be supporting, through companion diagnostic [68Ga]FAPI-46 PET, Yantai LNC Biotechnology’s recently FDA accepted clinical study. On Jan 6th, 2023 Yantai LNC Biotechnology received FDA clearance to [...]